Department of Pharmacology & Therapeutics, College of Medicine, Shaqra University, Saudi Arabia Email: dr.aslam@su.edu.sa
FDA on 13/09/2019 alerted patients and health care professionals to Glenmark Pharmaceutical Inc.’s voluntary recall of prescription ranitidine tablets (150 mg and 300 mg). The medicines are being recalled because they may contain unacceptable levels of N-nitrosodimethylamine (NDMA). FDA has advised companies on 17/12/2019 to recall their ranitidine if testing shows levels of NDMA above the acceptable daily intake (96 nanograms per day or 0.32 parts per million for ranitidine). The agency posted the results of its testing of ranitidine samples and has asked companies to conduct their own laboratory testing. Patients taking prescription ranitidine who wish to stop should talk to their health care professional about other treatment options. Multiple drugs are approved for the same or similar uses as ranitidine. FDA recalled the impured Ranitidine, but what about the human consumers who ate the carcinogenic impured Ranitidine by paying to Pharma companies?
Keywords: Ranitidine, N-nitrosodimethylamine impurities, cancer, acidity, H2 blocker
Ranitidine is an over-the-counter (OTC) and prescription drug. Ranitidine is an H2 (histamine-2) blocker, which decreases the amount of acid created by the stomach. Over-the-counter ranitidine is approved to prevent and relieve heartburn associated with acid ingestion and sour stomach. Prescription ranitidine is approved for multiple indications, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease.
Ranitidine Hydrochloride (Zantac tablet) approved by the US FDA on 29/10/1999. Approval was given to Glaxo Wellcome Research and Development.
As the FDA and other agencies around the world continue to investigate ranitidine, more details will become available. In the meantime, the FDA is not calling for individuals to stop taking the medication. However, for many conditions, ranitidine is only recommended for short-term use. If you have been using ranitidine for a while, now would be a good time to discuss with your physician whether you still need it and whether you might benefit from alternative treatment options, including other drug classes or a different H2 blocker.[1]
NDMA
NDMA is classified as a probable human carcinogen (a substance that could cause cancer) based on results from laboratory tests. NDMA is a known environmental contaminant typically found in water and foods, including meats, dairy products, and vegetables.
On 17/12/2019, Glenmark Pharmaceuticals Inc., USA Voluntarily Recalls All Unexpired Lots of its Ranitidine Tablets and Ceases Distribution, Due to Possible Presence of N-nitrosodimethylamine (NDMA) Impurity.
The recalled lots of Ranitidine Tablets 150 mg and 300 mg, which are listed in Attachment B, are being recalled because of the presence or potential presence of N-nitrosodimethylamine (NDMA) levels above the acceptable daily intake levels established by the FDA, based on FDA-validated tests.
Glenmark’s Ranitidine Tablets 150 mg and 300 mg are manufactured at two approved manufacturing facilities. Of the 928 recalled lots of Ranitidine Tablets, USP, 16 lots were manufactured by Glenmark Pharmaceuticals Ltd., Goa, India and 912 lots were manufactured by Strides Pharma Science Limited, Puducherry, India. 150 mg and 300 mg is a prescription oral product approved for multiple indications, including treatment and prevention of ulcers of the stomach and intestines and treatment of gastroesophageal reflux disease.
The Ranitidine Tablets, USP subject to the recall can be identified by the NDC number on the product label. The following NDCs of Ranitidine Tablets, USP, 150 mg and 300 mg, are included in this recall:
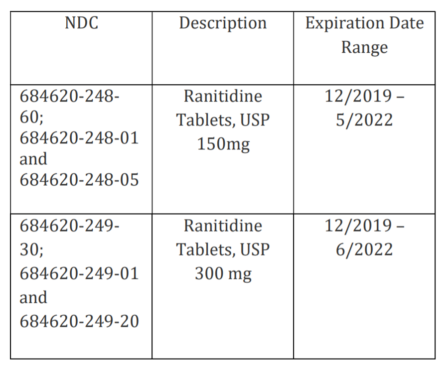
The affected Ranitidine Tablets were distributed directly to Wholesalers, Distributors, Retailers, and Repackagers nationwide. The 150mg products are packaged in bottle packs of 60’s, 100’s and 500’s, whereas, the 300mg products are packaged in bottle packs of 30’s, 100’s and 250’s.
FDA has determined that the levels of NDMA in Ranitidine and Nizatidine are similar to the levels you would expect to be exposed to if you ate common foods like grilled or smoked meats.[1]
Pharmaceutical companies paying millions of salaries every month to employers from the top-level scientist to the bottom level workers. The big question is that how impurities which may cause cancer detected in medicines? Pharmaceutical companies safely and easily give the statement like no adverse effect is detected at the consumer level with regards to detected impurity (To date, Glenmark has not received any reports of adverse events that have been confirmed to be directly related to this recall). Ranitidine is an OTC product consumed by the common man for the acidity. In most countries common man unable to get the diagnosis and treatment for cancer due to cost. How the common man report to Glenmark about cancer detected in him because of NDMA impurity? It’s not easy to diagnose cancer due to NDMA impurity and costeffective. FDA recalled the impured medicines, but what about the human consumers who ate the carcinogenic impured medicines by paying to Pharma companies?
Consumers who have Ranitidine Tablets, USP subject to this recall should immediately discontinue use and consult with their physician or healthcare provider about treatment options.
References
- 1.FDA. gov, Glenmark Pharmaceuticals Inc., USA Voluntarily Recalls All Unexpired Lots of its Ranitidine Tablets and Ceases Distribution, Due to Possible Presence of N-nitrosodimethylamine (NDMA) Impurity. updated 2019 17, Dec. available from https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/glenmark-pharmaceuticals-inc-usa-voluntarily-recalls-all-unexpired-lots-its-ranitidine-tablets-and [Google Scholar] [PubMed]

