Dr. Aslam Pathan Department of Pharmacology, College of Medicine, Shaqra University, Saudi ArabiaEmail: dr.aslam@su.edu.sa
The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has claimed a global health crisis of unpredictable amount. Due to its high mortality, many COVID-19 vaccines are being developed, approved, and manufactured rapidly. However, some serious neurological adverse events (AEs) were reported after the application of them. This review was conducted to collect and discuss published data about neurological side effects of SARS-CoV-2 vaccines in order to discover type, frequency, treatment, and outcome of these side effects. The most frequent neurological side effects of SARS-CoV-2 vaccines are headache, Guillain-Barre syndrome (GBS), venous sinus thrombosis (VST), and transverse myelitis. Healthcare practitioners, particularly neurologists involved in the management of patients having undergone SARS-CoV-2 vaccinations, should be aware of these side effects and should recognize them early and treat them significantly. However, some neurological conditions like headache and GBS are found in patients with COVID-19 infection and patients after the SARS-CoV-2 vaccinations. Further studies should be warranted to differentiate the etiology.
Keywords adverse effects, neuropathy, vaccination, COVID-19, SARS-CoV-2
According to the World Health Organization (WHO), more than 143 vaccine candidates are currently in clinical trials, and 9 of them (AstraZeneca/Oxford Vaccine, Johnson and Johnson, Moderna, Pfizer/BioNTech, Sinopharm, Sinovac, Bharat Biotech BBV152 COVAXIN, Covovax, and Nuvaxovid) have been granted emergency use authorization. However, during the past few months, serious adverse effects such as cerebral venous sinus thrombosis (CVST), splanchnic vein thrombosis, and thrombocytopenia were reported after vaccinations, and some cases have even proved fatal.[1],[2] The earliest reports from Wuhan found that 36.4% of patients showed a certain extent of neurological involvement, which included central nervous system (CNS) manifestations (dizziness, headache, impaired consciousness, acute cerebrovascular disease, and epilepsy), peripheral nervous system (PNS) manifestations (anosmia, hypogeusia, visual impairment, and neuralgia), and skeletal muscular damage.[3]
In a cohort study of 3,744 patients with clinically diagnosed or laboratory-confirmed COVID-19 at 28 centers, neurological manifestations were found in approximately 80% of patients; the most common self-reported symptoms included headache (37%) and anosmia or ageusia (26%), whereas the most common neurological signs and/or syndromes were acute encephalopathy (49%), coma (17%), and stroke (6%).[4]
More seriously, some of the above symptoms still bothered many survivors 1 year after discharge from hospitals, 10.4% suffered from anxiety, 2.3% from headache, 1.4% from taste change, and 1.3% from impaired sense of smell. Recently, by searching literature on thyroid dysfunction in patients with COVID-19, the entire hypothalamic-pituitary-thyroid axis could be the target of damage by COVID-19. Specifically, it could manifest as thyrotoxicosis or hypothyroidism.[5],[6]
A literature search in the databases PubMed using the search terms “SARS-CoV-2 Vaccination,” “side effects,” “adverse reactions,” and “neurological,” was conducted for the period December 2022 to February 2023. Initially detected were 80 titles in PubMed. Included were only original articles which reported a neurological adverse reaction. Excluded were articles that were repetitive and articles in which a causal relation between the vaccination and the complication could not be convincingly established. All approved vaccines were considered.
Fourteen case studies reported the neurological adverse effects and identified them in mass vaccination campaigns (Table 1). The vaccine AZD 1222 reported Guillain-Barré syndrome,[7][8][9][10][11] cerebral venous sinus thrombosis,[12][13][14][15][16][17] and transverse myelitis.[18][19][20][21][22] The vaccine Ad26.COV2.S reported cerebral venous sinus thrombosis,[23][24] and transverse myelitis.[25] The vaccine mRNA-1273 reported Bell’s palsy,[26][27][28][29] cerebral venous thrombosis,[30] Parsonage-Turner syndrome.[31][32] The vaccine BNT162b2 reported Bell’s palsy,[33] Guillain-Barré syndrome,[34] cerebral venous thrombosis,[30] small fiber neuropathy,[35] Parsonage-Turner syndrome,[31][32] and Delirium.[36] The vaccine AstraZeneca and Pfizer reported headaches in the 3051 patients.[37][38]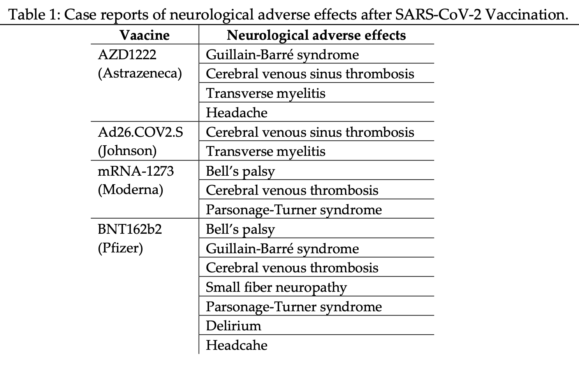
The most frequent neurological side effects of SARS‐CoV‐2 vaccines are headache, Guillain-Barré syndrome, venous sinus thrombosis, and transverse myelitis as per this review. Other neurological side effects occur at a very low frequency. The most common neurological adverse effect of SARS-CoV-2 vaccination is headache, which can occur with any of the licensed vaccines. The majority of the time, the headache begins within a few hours following the vaccine and subsides spontaneously within 48 hours.[37] The pathophysiological mechanism of Guillain-Barré syndrome after SARSCoV2 vaccination is unknown, however, molecular mimicry is thought to be the most feasible theory. Given that SARS-CoV-2 vaccines induce immunization against the spike protein and that the SARS-CoV-2 spike protein can bind to sialic acid-containing glycoproteins and gangliosides on cell surfaces, an antibody cross-reaction appears to be the most likely causal link between Guillain-Barré syndrome and SARSCoV2 immunization.[39] The third most common consequence of SARS-CoV-2 vaccines is venous sinus thrombosis, which is explained by hypercoagulability. The activation of platelets by the virus, which shifts endothelium from an antithrombotic to a prothrombotic state, and direct activation of complement pathways, which promotes thrombin production, have been linked to hypercoagulability following a SARSCoV2 vaccine.[40] The headache and GBS were also reported as neurological complications in the patients with COVID-19 infection.[41]
This study shows that safety concerns against SARS‐CoV‐2 vaccines are backed by an increasing number of studies reporting neurological side effects. The most frequent of them are headache, Guillain-Barré syndrome, venous sinus thrombosis, and transverse myelitis. Healthcare practitioners, particularly neurologists, involved in caring for patients who have received SARS-CoV-2 vaccinations should be aware of these side effects, recognize them early, and treat them significantly. However, some neurological conditions like headache and Guillain-Barré syndrome are found in patients with COVID-19 infections and patients after the SARS-CoV-2 vaccinations, further studies should be warranted to differentiate the etiology.
The authors declare no conflicts of interest relevant to this article.
References
- 1.Chen F, Cao P, Liu H, Cai D. The Impact of COVID-19 and Vaccine on the Human Nervous System. Neuroendocrinology. 2022;112(11):1046-1057. [Google Scholar] [PubMed]
- 2.Mehta PR, Apap Mangion S, Benger M, Stanton BR, Czuprynska J, Arya R, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination: a report of two UK cases. Brain Behav Immun. 2021 Jul;95:514–7. [Google Scholar] [PubMed]
- 3.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Jun 1;77((6)):683–90. [Google Scholar] [PubMed]
- 4.Chou SH, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19: a report for the GCS-NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021 May 3;4((5)):e2112131. [Google Scholar] [PubMed]
- 5.Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021 Sep 1;4((9)):e2127403. [Google Scholar] [PubMed]
- 6.Scappaticcio L, Pitoia F, Esposito K, Piccardo A, Trimboli P. Impact of COVID-19 on the thyroid gland: an update. Rev Endocr Metab Disord. 2021 Dec;22((4)):803–15. [Google Scholar] [PubMed]
- 7.Min YG, Ju W, Ha YE, Ban JJ, Lee SA, Sung JJ, et al. Sensory Guillain-Barre syndrome following the ChAdOx1 nCov-19 vaccine: report of two cases and review of literature. J Neuroimmunol. 2021 Aug 8;359:577691. [Google Scholar] [PubMed]
- 8.Keh RYS, Scanlon S, Datta-Nemdharry P, et al. COVID-19 vaccination and Guillain-Barré syndrome: analyses using the National Immunoglobulin Database. Brain. 2023;146(2):739-748. [Google Scholar] [PubMed]
- 9.Kim JE, Park J, Min YG, Hong YH, Song TJ. Associations of Guillain-Barré syndrome with coronavirus disease 2019 vaccination: Disproportionality analysis using the World Health Organization pharmacovigilance database. J Peripher Nerv Syst. 2022;27(3):206-214. [Google Scholar] [PubMed]
- 10.Lee HY, Lien WC. Effects of COVID-19 vaccine type on Guillain-Barré syndrome: Two cases and a literature review. Hum Vaccin Immunother. 2023;19(1):2171231. [Google Scholar] [PubMed]
- 11.Abolmaali M, Rezania F, Behnagh AK, Hamidabad NM, Gorji A, Mirzaasgari Z. Guillain-Barré syndrome in association with COVID-19 vaccination: a systematic review. Immunol Res. 2022;70(6):752-764. [Google Scholar] [PubMed]
- 12.Krzywicka K, Heldner MR, Sanchez van Kammen M, van Haaps T, Hiltunen S, Silvis SM, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021 Nov;28((11)):3656–62. [Google Scholar] [PubMed]
- 13.Krzywicka K, van de Munckhof A, Sánchez van Kammen M, et al. Age-Stratified Risk of Cerebral Venous Sinus Thrombosis After SARS-CoV-2 Vaccination. Neurology. 2022;98(7):e759-e768. [Google Scholar] [PubMed]
- 14.Wittstock M, Walter U, Volmer E, Storch A, Weber MA, Großmann A. Cerebral venous sinus thrombosis after adenovirus-vectored COVID-19 vaccination: review of the neurological-neuroradiological procedure. Neuroradiology. 2022;64(5):865-874. [Google Scholar] [PubMed]
- 15.Donaldson L, Qian J, Lutchman C, AlShafai L, Margolin E. Cerebral Venous Sinus Thrombosis and Papilledema in Vaccine-Induced Thrombotic Thrombocytopenia After SARS-CoV-2 Vaccination. J Neuroophthalmol. 2023;43(1):e16-e18. [Google Scholar] [PubMed]
- 16.Palaiodimou L, Stefanou MI, Katsanos AH, et al. Cerebral Venous Sinus Thrombosis and Thrombotic Events After Vector-Based COVID-19 Vaccines: A Systematic Review and Meta-analysis. Neurology. 2021;97(21):e2136-e2147. [Google Scholar] [PubMed]
- 17.Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci. 2021;428:117607. [Google Scholar] [PubMed]
- 18.Pagenkopf C, Südmeyer M. A case of longitudinally extensive transverse myelitis following vaccination against Covid-19. J Neuroimmunol. 2021 Sep 15;358:577606. [Google Scholar] [PubMed]
- 19.Notghi AA, Atley J, Silva M. Lessons of the month 1: Longitudinal extensive transverse myelitis following AstraZeneca COVID-19 vaccination. Clin Med (Lond). 2021;21(5):e535-e538. [Google Scholar] [PubMed]
- 20.Hsiao YT, Tsai MJ, Chen YH, Hsu CF. Acute Transverse Myelitis after COVID-19 Vaccination. Medicina (Kaunas). 2021;57(10):1010. [Google Scholar] [PubMed]
- 21.Khan E, Shrestha AK, Colantonio MA, Liberio RN, Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J Neurol. 2022;269(3):1121-1132. [Google Scholar] [PubMed]
- 22.Maroufi SF, Naderi Behdani F, Rezania F, Tanhapour Khotbehsara S, Mirzaasgari Z. Longitudinally extensive transverse myelitis after Covid-19 vaccination: case report and review of literature. Hum Vaccin Immunother. 2022;18(1):2040239. [Google Scholar] [PubMed]
- 23.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021 Jun 22;325((24)):2448–56. [Google Scholar] [PubMed]
- 24.Karron RA, Key NS, Sharfstein JM. Assessing a Rare and Serious Adverse Event Following Administration of the Ad26.COV2.S Vaccine. JAMA. 2021;325(24):2445-2447. [Google Scholar] [PubMed]
- 25.Tahir N, Koorapati G, Prasad S, Jeelani HM, Sherchan R, Shrestha J, et al. SARS-CoV-2 vaccination-induced transverse myelitis. Cureus. 2021 Jul;13((7)):e16624. [Google Scholar] [PubMed]
- 26.Martin-Villares C, Vazquez-Feito A, Gonzalez-Gimeno MJ, de la Nogal-Fernandez B. Bell's palsy following a single dose of mRNA SARS-CoV-2 vaccine: a case report. J Neurol. 2022;269(1):47-48. [Google Scholar] [PubMed]
- 27.Cellina M, D'Arrigo A, Floridi C, Oliva G, Carrafiello G. Left Bell's palsy following the first dose of mRNA-1273 SARS-CoV-2 vaccine: A case report. Clin Imaging. 2022;82:1-4. [Google Scholar] [PubMed]
- 28.Iftikhar H, Noor SMU, Masood M, Bashir K. Bell's Palsy After 24 Hours of mRNA-1273 SARS-CoV-2 Vaccine. Cureus. 2021;13(6):e15935. [Google Scholar] [PubMed]
- 29.Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64-72. [Google Scholar] [PubMed]
- 30.Abbattista M, Martinelli I, Peyvandi F. Comparison of adverse drug reactions among four COVID-19 vaccines in Europe using the EudraVigilance database: thrombosis at unusual sites. J Thromb Haemost. 2021;19((10)):2554–8. [Google Scholar] [PubMed]
- 31.Queler SC, Towbin AJ, Milani C, Whang J, Sneag DB. Parsonage-Turner syndrome following COVID-19 vaccination: MR neurography. Radiology. 2022 Jan;302((1)):84–7. [Google Scholar] [PubMed]
- 32.Cassart EM, Vilas DR, Abe R, Cavanilles-Walker JM. Parsonage-Turner Syndrome After COVID-19 Vaccination in a Child. J Am Acad Orthop Surg Glob Res Rev. 2023;7(3):e22.00156. [Google Scholar] [PubMed]
- 33.Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021 Oct;268((10)):3589–91. [Google Scholar] [PubMed]
- 34.Razok A, Shams A, Almeer A, Zahid M. Post-COVID-19 vaccine Guillain-Barré syndrome; first reported case from Qatar. Authorea. 2021 Jul;67:102540. [Google Scholar] [PubMed]
- 35.Waheed W, Carey ME, Tandan SR, Tandan R. Post COVID-19 vaccine small fiber neuropathy. Muscle Nerve. 2021 Jul;64((1)):E1–2. [Google Scholar] [PubMed]
- 36.Zavala-Jonguitud LF, Perez-Garcia CC. Delirium triggered by COVID-19 vaccine in an elderly patient. Geriatr Gerontol Int. 2021 Jun;21((6)):540. [Google Scholar] [PubMed]
- 37.Göbel CH, Heinze A, Karstedt S, et al. Clinical characteristics of headache after vaccination against COVID‐19 (coronavirus SARS‐CoV‐2) with the BNT162b2 mRNA vaccine: a multicentre observational cohort study. Brain Commun. 2021;3(3):fcab169. [Google Scholar] [PubMed]
- 38.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979‐1993. [Google Scholar] [PubMed]
- 39.Introna A, Caputo F, Santoro C, et al. Guillain‐Barré syndrome after AstraZeneca COVID‐19‐vaccination: a causal or casual association? Clin Neurol Neurosurg. 2021;208:106887. [Google Scholar] [PubMed]
- 40.Steadman E, Fandaros M, Yin W. SARS‐CoV‐2 and plasma hypercoagulability. Cell Mol Bioeng. 2021;28:1‐10. [Google Scholar] [PubMed]
- 41.Pathan A. Neurological complications and COVID-19: A literature review. NeuroPharmac J. 2021; 6(1): 142-144. [Google Scholar] [PubMed]
