Department of Pharmacology, College of Medicine, Shaqra University, Shaqra-11961, Saudi Arabia.
Alzheimer disease (AD) is a neurodegenerative disorder marked by cognitive and behavioral impairment that significantly interferes with social and occupational functioning. AD is a progressive and fatal dementia of unknown cause characterized by loss of cognitive and physical functioning, commonly with behavior or cognitive symptoms. Cognitive decline is gradual and includes memory loss, aphasia, apraxia, agnosia, disorientation, and impaired executive function. physicians will often play a major role in diagnosing and treating dementia and related disorders in the community. Accurate treatment of cognitive symptoms is important. Accordingly, we reviewed the available pharmacotherapy in the clinical management of cognitive symptoms of dementia.
Cognitive decline is gradual and includes memory loss, aphasia, apraxia, agnosia, disorientation, and impaired executive function. Other symptoms include depression, psychotic symptoms, aggression, motor hyperactivity, uncooperativeness, wandering, and combativeness. Patients become increasingly unable to care for themselves. Subjective cognitive decline (SCD) is common in older adults and may be an early marker of future cognitive decline. Research suggest that SCD is more closely related to concurrent symptoms of depression.[1], [3] Subjective cognitive decline is indicative of accumulation of early tauopathy in the medial temporal lobe, specifically in the entorhinal cortex, and to a lesser extent, elevated global levels of Aβ. Our findings suggest multiple underlying pathways that motivate SCD that do not necessarily interact to influence SCD endorsement. As such, multiple biological factors must be considered when assessing SCD in clinically healthy older adults.[2], [4] Research increasingly suggests that subjective cognitive decline (SCD) in older adults, in the absence of objective cognitive dysfunction or depression, may be a harbinger of non-normative cognitive decline and eventual progression to dementia.[5] suggest that depressive symptoms, particularly anhedonia and negative affect, are related to cognitive decline in older African Americans.[6], [8] Subjective memory complaints (SMCs) represent a type of complaint made by individuals with cognitive symptoms or complaints but no clear impairment on objective psychometric testing.[9], [12] Evidence suggests that individuals with SMCs may be at increased risk of dementia, with several studies showing that patients with cognitive complaints have a higher rate of progression to mild cognitive impairment (MCI) or AD than those without.[13], [18] Subjective memory impairment (SMI) is receiving increasing attention as a pre-mild cognitive impairment (MCI) condition in the course of the clinical manifestation of Alzheimer disease (AD).[19] The prediction of dementia in AD by SMI with subsequent amnestic MCI supports the model of a consecutive 3-stage clinical manifestation of AD from SMI via MCI to dementia.[20] , [21] The diagnostic criteria of the National Institute on Aging and the Alzheimer’s Association view AD as a spectrum beginning with an asymptomatic preclinical phase progressing to the symptomatic preclinical phase and then to the dementia phase. AD is a clinical diagnosis, based largely on identified symptoms and difficulty with activities of daily living revealed by patient and caregiver interviews. The Folstein Mini-Mental State Examination (MMSE) can help establish a history of deficits in two or more areas of cognition at baseline against which to evaluate change in severity over time (table 1). The average expected decline in an untreated patient is 2 to 4 points per year.

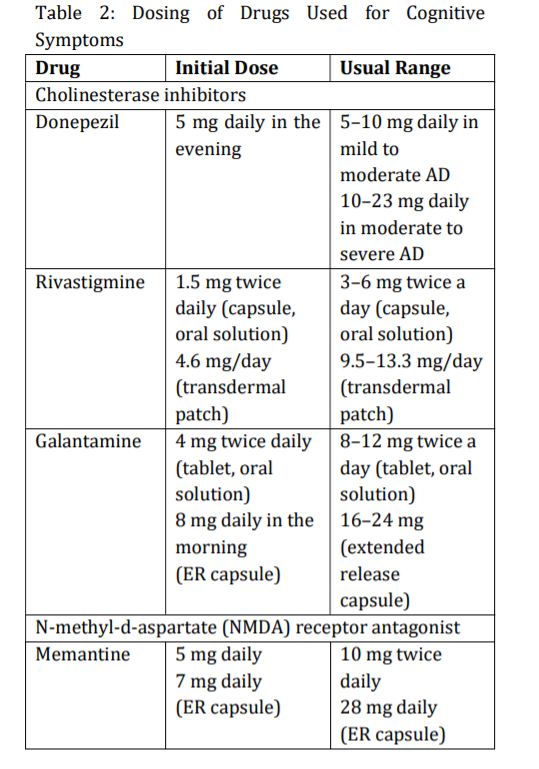
The mainstay of therapy for patients with Alzheimer disease (AD) is the use of centrally acting cholinesterase inhibitors to attempt to compensate for the depletion of acetylcholine (ACh) in the cerebral cortex and hippocampus. A partial Nmethyl-D-aspartate (NMDA) antagonist is approved for treatment of moderate and severe AD. Table 2 summarizes dosing of the cholinesterase inhibitors and memantine. When switching from one cholinesterase inhibitor to another, 1 week washout is generally sufficient. No comparative trials have assessed the effectiveness of one agent over another. Donepezil, rivastigmine, and galantamine are indicated in mild to moderate AD; donepezil is also indicated for severe AD.[22] , [23]
If the decline in MMSE score is more than 2 to 4 points after treatment for 1 year with the initial agent, it is reasonable to change to a different cholinesterase inhibitor. Otherwise, treatment should be continued with the initial medication throughout the course of the illness. The three cholinesterase inhibitors have similar efficacy in mild to moderate AD, and duration of benefit lasts 3 to 12 months. Because of their short half-lives, if rivastigmine or galantamine treatment is interrupted for several days or longer, retitrate starting at the lowest dose. The most frequent adverse effects include mild to moderate gastrointestinal (GI) symptoms (nausea, vomiting, and diarrhea), urinary incontinence, dizziness, headache, syncope, bradycardia, muscle weakness, salivation, and sweating. Abrupt discontinuation can cause worsening of cognition and behavior in some patients. [24] , [29]
Memantine (Namenda) blocks glutamatergic neurotransmission by antagonizing N-methyl-daspartate receptors, which may prevent excitotoxic reactions. It is used as monotherapy and in combination with a cholinesterase inhibitor. It is indicated for treatment of moderate to severe AD, but not for mild AD. It is not metabolized by the liver but is primarily excreted unchanged in the urine. Dosing must be adjusted in patients with renal impairment. It is usually well tolerated; side effects include constipation, confusion, dizziness, and headache. Because of the incidence of side effects and a lack of supporting evidence, neither NSAIDs nor prednisone is recommended for treatment or prevention of AD. Because of limited efficacy data, the potential for adverse effects (eg, nausea, vomiting, diarrhea, headache, dizziness, restlessness, weakness, and hemorrhage), and poor standardization of herbal products, ginkgo biloba is not recommended for prevention or treatment of AD.[30] ,[33]
Fixed dose combination capsule containing memantine extended-release and donepezil for patients with moderate-to-severe Alzheimer disease currently stabilized on donepezil 10 mg once daily. Administer once daily in the evening. Starting dose is 7 mg/10 mg PO, taken once a day in the evening Increase dose in 7 mg increments based on the memantine component to the recommended maintenance dose of 28 mg/10 mg once daily. [34]
Medical foods are dietary supplements intended to compensate specific nutritional problems caused by a disease or condition. Caprylidene is a prescription medical food that is metabolized into ketone bodies. The brain can use these ketone bodies for energy when its ability to process glucose is impaired, which brain-imaging scans suggest is the case in AD. [35]
References
- 1.Zlatar ZZ, Muniz MC, Espinoza SG, et al. Subjective Cognitive Decline, Objective Cognition, and Depression in Older Hispanics Screened for Memory Impairment. J Alzheimers Dis. 2018;63(3):949–956. [Google Scholar] [PubMed]
- 2.Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, et. al. RegionSpecific Association of Subjective Cognitive Decline With Tauopathy Independent of Global β-Amyloid Burden. JAMA Neurol. 2017;74(12):1455-1463. [Google Scholar] [PubMed]
- 3.Pathan A, Alshahrani A. Alzheimer’s Disease: Pharmacotherapy of noncognitive symptoms. NeuroPharmac J. 2018; 3(1): 47-50. [Google Scholar] [PubMed]
- 4.Markova H, Andel R, Stepankova H, Kopecek M, Nikolai T, Hort J, et. al. Subjective Cognitive Complaints in Cognitively Healthy Older Adults and Their Relationship to Cognitive Performance and Depressive Symptoms. J Alzheimers Dis. 2017;59(3):871-881. [Google Scholar] [PubMed]
- 5.Rabin LA, Smart CM, Crane PK. Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures Used Across 19 International Research Studies. J Alzheimers Dis. 2015;48 Suppl 1:S63-86. [Google Scholar] [PubMed]
- 6.Turner AD, Capuano AW, Wilson RS, Barnes LL. Depressive symptoms and cognitive decline in older african americans: two scales and their factors. Am J Geriatr Psychiatry. 2015;23(6):568-78. [Google Scholar] [PubMed]
- 7.Guerrero-Berroa E, Kluger A, Schmeidler J, Sailor K, Lizardi H, et. al. Neuropsychological and neuropsychiatric prediction of global cognitive status among older Spanishspeaking Hispanics and English-speaking whites. J Geriatr Psychiatry Neurol. 2014;27(4):266-75 [Google Scholar] [PubMed]
- 8.Farias ST, Mungas D, Hinton L, Haan M. Demographic, neuropsychological, and functional predictors of rate of longitudinal cognitive decline in Hispanic older adults. Am J Geriatr Psychiatry. 2011;19(5):440-50. [Google Scholar] [PubMed]
- 9.Steinberg SI, Negash S, Sammel MD, Bogner H, Harel BT, et. al. Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am J Alzheimers Dis Other Demen. 2013;28(8):776-83. [Google Scholar] [PubMed]
- 10.Montejo Carrasco P, Montenegro-Pena M, Lopez-Higes R, Estrada E, et. al. Subjective Memory Complaints in healthy older adults: Fewer complaints associated with depression and perceived health, more complaints also associated with lower memory performance. Arch Gerontol Geriatr. 2017;70:28-37. [Google Scholar] [PubMed]
- 11.Ryu SY, Lee SB, Kim TW, Lee TJ. Subjective memory complaints, depressive symptoms and instrumental activities of daily living in mild cognitive impairment. Int Psychogeriatr. 2016;(3):487-94. [Google Scholar] [PubMed]
- 12.Gallagher D, Kiss A, Lanctot K, Herrmann N. Depressive symptoms and cognitive decline: A longitudinal analysis of potentially modifiable risk factors in community dwelling older adults. J Affect Disord. 2016;190:235-240. [Google Scholar] [PubMed]
- 13.Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes RD, et. al. Subjective Cognitive Complaints, Personality and Brain Amyloidbeta in Cognitively Normal Older Adults. Am J Geriatr Psychiatry. 2015;(9):985-93. [Google Scholar] [PubMed]
- 14.Maki Y, Yamaguchi T, Yamagami T, Murai T, Hachisuka K, et. al. The impact of subjective memory complaints on quality of life in community-dwelling older adults. Psychogeriatrics. 2014;14(3):175-81. [Google Scholar] [PubMed]
- 15.Chin J, Oh KJ, Seo SW, Na DL. Are depressive symptomatology and self-focused attention associated with subjective memory impairment in older adults? Int Psychogeriatr. 2014;26(4):573-80. [Google Scholar] [PubMed]
- 16.Balash Y, Mordechovich M, Shabtai H, Giladi N, Gurevich T, Korczyn AD. Subjective memory complaints in elders: depression, anxiety, or cognitive decline? Acta Neurol Scand. 2013;127(5):344-50. [Google Scholar] [PubMed]
- 17.Slavin MJ, Brodaty H, Kochan NA, Crawford JD, Trollor JN, Draper B, Sachdev PS. Prevalence and predictors of “subjective cognitive complaints” in the Sydney Memory and Ageing Study. Am J Geriatr Psychiatry. 2010;18(8):701-10. [Google Scholar] [PubMed]
- 18.Cook, S. and Marsiske, M. Subjective memory beliefs and cognitive performance in normal and mildly impaired older adults. Aging and Mental Health. 2006;10:413–423. [Google Scholar] [PubMed]
- 19.Jessen, F. et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Archives of General Psychiatry. 2010;67:414–422. [Google Scholar] [PubMed]
- 20.Gifford, K. A. et al. Subjective memory complaint only relates to verbal episodic memory performance in mild cognitive impairment. Journal of Alzheimer’s Disease. 2015;44:309–318. [Google Scholar] [PubMed]
- 21.Abdulrab, K. and Heun, R. (2008). Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. European Psychiatry. 2008;23:321–330. [Google Scholar] [PubMed]
- 22.Yaffe K, Boustani M. Benzodiazepines and risk of Alzheimer’s disease. BMJ. 2014;349: 5312. [Google Scholar] [PubMed]
- 23.Bredesen DE. Reversal of cognitive decline: A novel therapeutic program. Aging (Albany NY). 2014; 6:707-717. [Google Scholar] [PubMed]
- 24.Small GW. Treating dementia and agitation. JAMA. 2014;311(7):677-8. [Google Scholar] [PubMed]
- 25.Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol. 2012;73(4):504–517. [Google Scholar] [PubMed]
- 26.Kavanagh S, Gaudig M, Van Baelen B, et al. Galantamine and behavior in Alzheimer disease: analysis of four trials. Acta Neurol Scand. 2011;124(5):302-8. [Google Scholar] [PubMed]
- 27.Farlow M, Veloso F, Moline M, et al. Safety and tolerability of donepezil 23 mg in moderate to severe Alzheimer’s disease. BMC Neurol. 2011;11:57. [Google Scholar] [PubMed]
- 28.Starr JM. Cholinesterase inhibitor treatment and urinary incontinence in Alzheimer’s disease. J Am Geriatr Soc. 2007;55(5):800-1. [Google Scholar] [PubMed]
- 29.Gill SS, Anderson GM, Fischer HD, Bell CM, Li P, Normand SL, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867-73. [Google Scholar] [PubMed]
- 30.Lachaine J, Beauchemin C, Legault M, Bineau S. Economic evaluation of the impact of memantine on time to nursing home admission in the treatment of Alzheimer disease. Can J Psychiatry. 2011;56(10):596- 604. [Google Scholar] [PubMed]
- 31.Schmitt FA, van Dyck CH, Wichems CH, Olin JT. Cognitive response to memantine in moderate to severe Alzheimer disease patients already receiving donepezil: an exploratory reanalysis. Alzheimer Dis Assoc Disord. 2006;20(4):255-62. [Google Scholar] [PubMed]
- 32.Porsteinsson AP, Grossberg GT. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83-9. [Google Scholar] [PubMed]
- 33.Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011;68(8):991-8. [Google Scholar] [PubMed]
- 34.Greig SL. Memantine ER/Donepezil: A Review in Alzheimer’s Disease. CNS Drugs. 2015;29 (11):963-70. [Google Scholar] [PubMed]
- 35.Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, doubleblind, placebo-controlled, multicenter trial. Nutr Metab (Lond). 2009;6:31. [Google Scholar] [PubMed]