Department of Bioengineering, Integral University, Lucknow-226026, Uttar Pradesh, India
ABSTRACT: In the current era, plastic has become a global environmental menace affecting terrestrial and aquatic ecosystems. Regular plastic resilient nature towards decomposition, and it pollutes the environment. Conventional plastic is widely used in various industrial setups with no alternate substitute available. The quest to find an alternate solution to the emerging problem development of bioplastic that is eco-friendlier and adds no pollution to the environment has been much focussed. Bioplastic is plastic synthesized from renewable biomass sources rather than petroleum origin. The development of bioplastic of microbial origin will be a promising innovation to keep our world plastic-free and promote sustainability. It can be degraded easily and gets broken down into carbon dioxide, biomass, and water rapidly. The present reviews highlight the sources of microbial-derived bioplastic, polyhydroxybutyrate (PHB), polyhydroxyalkanoates (PHAs), extraction methodologies, optimization strategies to improve yield, degradation, application areas, present challenges, and prospects in production. We have also provided a brief insight into gene and gene clusters responsible for bioplastic production. Overall, the article will provide a comprehensive update on bioplastic to help mitigate our current problem associated with conventional plastic usage.
Keywords: Bioplastic, PHBs, PHAs, classification of plastics, Degradation of bioplastics, PLAs
One of the 17 UN sustainable goals aims for a clean environment where bioplastic use could achieve sustainability to a great extent. Synthetic polymers are incredibly stable in the environment and do not degrade by biogeochemical cycles of the biosphere, which has to lead them to be a global pollutant and poses a threat to sustainable development in the world.[1] A primary environmental concern is a gradual accumulation of synthetic plastics, which are non-biodegradable, directly impacting global warming, eco-toxicity, ozone depletion, and soilwater pollution. Thus, it seeks to develop means to reduce plastic use by practicing bioplastics in dayto-day use. One aspect of incorporating PHA bioplastic production within plants with the aid of genetic manipulation could significantly link agriculture with the material industry’s demands. Sources of bioplastics range from a wide variety of renewable sources such as cellulose, starch, chitin, proteins, lipids, and majorly polymers from plant/microbial origin, i.e., PHAs and PHBs.[2] Polylactic acid has also shown great potential in bioplastic production apart from its other polymer like polycaprolactone, polyglycolic, PHB is used mainly in pharmaceutical applications.[3] Compared to petroleum-based plastics, degradation of bioplastic supports its sustainable development approach, which quickly gets degraded into carbon dioxide, water, and biomass under aerobic conditions. Compositing under microbial action is useful in degrading polylactic acid, PHA, or other plant-derived bioplastics like bio-polyethylene terephthalate and [1],[3]-propanediol[4], but PLA showed a slow degradation processing taking 11- month time for decomposition. Thus, to further enhance biodegradability, either the polymer’s blending could be an effective solution or improve the composting methodology.
Classification of Plastics
Based on biodegradability, plastic can be classified into biodegradable and non-biodegradable. Chemical structure and degradation patterns have been explored mainly for various plastics. Fossil fuel-based plastics are conventional plastic, nonbiodegradable due to their extensive repetition of monomer units and higher molecular weight.[5] The domain of non-biodegradable plastic includes PVC, PP, PS, PET, and PUR. Due to their harsh nature and poor waste management practices, it has posed a severe threat of pollution to the earth. Polyolefinsbased plastics are widely used in various domains of our day-to-day life; because of their highly stable nature in the environment, it is vital to look for proper waste management.[6] Although to enhance its degradability, starch and prooxidants are preferred for better fragmentation of plastics. The diverse range of bioplastic is summarized in Figure 1. While on the other hand, biodegradable bioplastics have a high biodegradability rate and get easily disintegrated by microbes’ action, thus possess a lesser threat to the ecosystem. Factors like polymer molecular weight, functional group, and crystallinity affect plastic’s degradation process.[8] Exoenzymes released by microbe break down these complex molecules into simpler ones using aerobic and anaerobic processes.[6],[9] Fungi and bacteria degrade plastics finally into carbon dioxide, and water through various metabolic activities, e.g.,strains like bacillus and Brevibacillus produce proteases with aid in the degradation of plastics.[10] Fungi are natural decomposers that produce laccase enzymes for the degradation of plastics.[11] Under the anaerobic situation, bacteria use oxygen as an electron donor to synthesize smaller organic molecules. While under anaerobic conditions in the absence of oxygen, other molecules like nitrate, sulfate, and manganese are used as electron acceptors.[12]
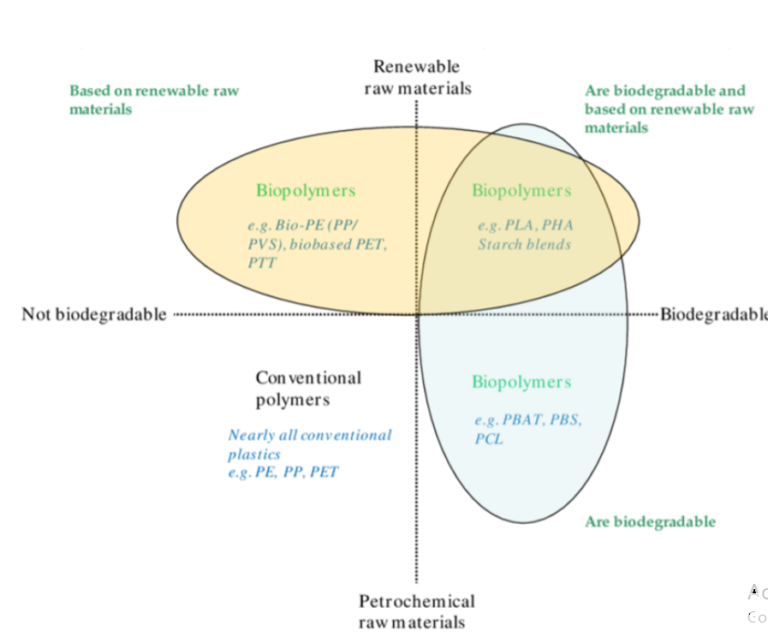
Figure 1: Types of bioplastics.[7]
Production of PHB and PHA from microbial sources
PHA are linear polyesters that comprise of molecular weight more than 60000 Daltons, which is commonly observed to get accumulated in a variety of microbes, including gram harmful bacteria, gram-positive bacteria, and some archaea as intracellular granules under unfavorable growth conditions.[13] ,[15]
PHB is the principal subsidiary of PHA. Under limited nutrient conditions with surplus carbon, various bacteria such as Alcaligene eutrophus, Azotobacter vinelandii, Bacillus, Streptomyces accumulate PHA as stored food material.[16] ,[18] The optimized strain of Rhizobium elti E1 and Pseudomonas stutzeri E114 improves the yield of PHBs when given mannitol as carbon and ammonium sulfate as a nitrogen source at PH of [7]. Optimum PHB production conditions were 48 h, temperature 30 oC under incubation period of 48 hrs during the highest yield.[19]
PHBs are biodegradable polyesters that have great potential to develop fully biodegradable plastics. The cheapest source for its production is agricultural products like cane molasses and steep corn liquor as carbon and nitrogen sources.[20] Fungi and bacteria utilize PHB, a thermoplastic polyester in intracellular carbon, and accumulate in the cell as an inclusion body.[21] Obtaining a cheap carbon source is a significant hurdle in the production of PHA and PHBs. It is estimated that one-tonne polymer 3 tons of glucose are needed, which is pretty costly. PHB can be obtained using other cheap sources like agro-residues, dairy waste, molasses, corn steep liquor.[22] ,[23]
Microalgae have appeared as rich sources for bioplastic production.[7] For bioplastic production, Chlorella and Spirulina species are the most promising microalgae.[77] Biomass produced by microalgae is potentially high, and it is not dependent upon food sources as substrate and has potential for high lipid accumulation.[24] ,[25] Bioplastic from microalgae offers a more sustainable approach and contributes to the bioeconomy.[26] ,[27] Growing microalgae in a closed cultivation system involve much lower cost with higher production.[28] optimizes phosphate buffer increased PHA production by fourfold.[30] , Bacterium Cupriavidus necator is known to accumulate PHB in a large amount. Using the Plackett-Burman design, crucial factors affecting the accumulation of PHB granules were agitation speed, fructose, KH2PO4, and initial pH, which yielded 7.46 g/L of PHB.[31] Similarly, for Botryococcus braunii applying RSM, the optimal parameters were pH in the range of 4-11, temperature 30-50 0C, and sewage water as a substrate for maximum yield of PHB was studied.[32] Likewise, various statistical optimization was employed for obtaining a maximum yield of PHB in Bacillus subtilis NG05, Burkholderia sacchari, Bacillus sphaericus NCIM 5149, with a variety of substrate.[33] ,[34]
Strain improvement and optimization of culture
A gram-positive bacterium SRKP-3, which resembled Bacillus megaterium, was identified from brackish water showing an excellent capability to accumulate PHAs. Optimization using response surface methodology resulted in an enhanced yield of 6.37 g/L of PHB dry weight at pH 9.22 Optimization of Alcaligenes sp. in batch culture was performed for PHB production using cane molasses and urea as a nutrient source. Strain selection was made using Nile blue A staining; parameter optimization showed 34.5 °C temperature, 6.54 pH, 3.13 Hz agitation enhanced peed to result in PHB yield of 76.80% of dry cell mass.[29] Yang et al., 2010 showed enhanced PHA production in Ralstonia eutropha H16 by using an optimal acetate, propionate, and butyrate dose. With increasing butyrate production, PHA production is enhanced by 1.5-fold, and further optimizes phosphate buffer increased PHA production by fourfold.[30] Bacterium Cupriavidus necator is known to accumulate PHB in a large amount. Using the Plackett-Burman design, crucial factors affecting the accumulation of PHB granules were agitation speed, fructose, KH2PO4, and initial pH, which yielded 7.46 g/L of PHB.[31] Similarly, for Botryococcus braunii applying RSM, the optimal parameters were pH in the range of 4-11, temperature 30-50 0C, and sewage water as a substrate for maximum yield of PHB was studied.[32] Likewise, various statistical optimization was employed for obtaining a maximum yield of PHB in Bacillus subtilis NG05, Burkholderia sacchari, Bacillus sphaericus NCIM 5149, with a variety of substrate.[33],[34]
Genetic approaches used in enhancing bioplastic production
Several archaea and eubacteria accumulate PHA within their cells to meet energy needs and carbon storage. The alteration in genetic and metabolic pathways for PHA metabolism has been achieved in these bacteria. However, finding a cost-effective production methodology remains a hurdle that could be sorted out using genetic engineering these microbes to produce PHAs in a high amount.[35] Earlier in 1990, Pseudomonas saccharophila and Alcaligenes eutrophus were engineered to uptake galactose and lactose, but they resulted in low PHB accumulation within the cell.[36] At the same time, incorporation of lacZ, lacl, and lacO genes from E. coli to C. necator allowed better lactose utilization resulting in higher accumulation.[37]Likewise, by introducing Mannheimia succiniciproducens MBEL55E sacC gene to R. eutropha for sucrose pathway utilization was engineered, which produced 83% of CDM in the defined medium.[38] Another recombinant that expressed Pseudomonas sp. 6–19 phaC1437, Clostridium propionicum pct540, was designed.[39] In the chemical mutagenesis approach, a mutant strain of C. necator was developed that produced 88% PHB using rapeseed oil.[40] The recombinant R. eutropha can utilize various plant oil as a substrate is well-reviewed, which leads to a yield of PHB in the range of 72 to 87% of CDM.41A. caviae based phaJ gene was inserted in the PHA biosynthesis operon that resulted in a higher PHA accumulation of 79% using soybean oil.[42] By cloning Pseudomonas lipase genes which metabolize triacylglycerols (TAGs) were introduced to enhance PHA accumulation.[43] Biosynthetic pathway poly-beta-hydroxybutyrate from Alcaligenes eutrophus H16 was cloned and expressed in E. coli for PHA production.[44] ,[45] Balaji et al., 201346 mentioned applying genetic modifications in a microorganism to produce PHB and its extraction process and have shown cyanobacteria’s potential as a PHB source by using alternate substrates. Bacterial genes were incorporated into the tobacco plastome to enhance PHB production using an operon extension strategy.[47] Likewise, transgenic cotton was used for PHB production. Deciphering the methylperillate biosynthesis pathway from Salvia dorisiana trichomes is another novel route for the biosynthesis of bioplastics from plants. The engineered enzymes for metabolic engineering could be used in biobased chemicals.[48] Thus, apart from using conventional techniques, recombinant technology could provide a significant boost to PHA production
Degradation of bioplastics
Current polymer degradation strategies rely on chemical, thermal, photo, and biological procedures. In proper waste management strategies coupled with industrially controlled biodegradation facilities, biodegradable plastics for some applications such as the packaging or health industry are promising and attractive options for economic, environmental, and health benefits.[49] Many studies have reported the striking ability of certain microorganisms, including bacteria and fungi, in fast degradation of both bio-based and fossil-based biodegradable polymers under stress conditions by producing exoenzymes and their products.[50] ,[53] Various microorganisms tend to degrade bioplastic and finally convert it to carbon dioxide and water via microbial metabolic activity. On the contrary, petrochemical plastic is resistant to microbial degradation.[54] Degradation of bioplastic to a greater extent, depends on the resident microbial population and begins once the colonization process is completed, leading to a lag phase.[55] The extent of biodegradability of biopolymers depends on their physical and chemical properties and their located environment, which is vital in their biodegradation.[56] The molecular structure of PLA, material properties, and environmental factors such as humidity, temperature, pH, microbes, and enzymes’ presence determine the degradation of bioplastics.[57] ,[58] Anaerobic conditions favor the degradation of polylactide, phosphinocarboxylic acid, and polyhydroxyalkanoates. Energy derived from mineralization is utilized by microbes and biomass.[59]
The extensive studies on the degradation aspect of bioplastic have deepened our understanding regarding degrading microbes and their enzymes. The knowledge will further enhance our understanding of designing biodegradable polymers. Besides, the blending of a biopolymer with natural biomaterials could be one way to reduce the high cost. Our knowledge on the subject will open new avenues towards the development of cost-effective greener bioplastic. Novel approaches for enhanced production of bioplastic and their integration in daily use will offer a greener alternate to petrol-based plastic and help attain sustainable development goals. Consequently, looking out for newer sources will further strengthen its applicability and ensure environmental safety and sustainability.
References
- 1.Shimao M. Biodegradation of plastics. Curr Opin Biotechnol. 2001;12(3):242–7. [Google Scholar] [PubMed]
- 2.Ponnaiah P, Shukri H, Muruganandham C, Narendrakumar G, Samrot A V. Utilization of palm oil mill effluent and clindamycin for optimization of polyhydroxy[r] alkanoates production. Biointerface Res Appl Chem. 2019 ;10(1):4740–6. [Google Scholar] [PubMed]
- 3.Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresour Technol. 2010;101(22):8493–501. [Google Scholar] [PubMed]
- 4.Kurdikar D, Fournet L, Slater SC, Paster M, Gruys KJ, Gerngross TU, et al. Greenhouse Gas Profile of a Plastic Material Derived from a Genetically Modified Plant. J Ind Ecol. 2000;4(3):107–22. [Google Scholar] [PubMed]
- 5.Ghosh SK, Pal S, Ray S. Study of microbes having potentiality for biodegradation of plastics. Environ Sci Pollut Res. 2013;20(7):4339–55. [Google Scholar] [PubMed]
- 6.Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: A comprehensive review. Biotechnol Adv. 2008;26(3):246–65. [Google Scholar] [PubMed]
- 7.Onen Cinar S, Chong ZK, Kucuker MA, Wieczorek N, Cengiz U, Kuchta K. Bioplastic Production from Microalgae: A Review. Int J Environ Res Public Health. 2020;17(11):3842. [Google Scholar] [PubMed]
- 8.Artham T, Doble M. Biodegradation of Aliphatic and Aromatic Polycarbonates. Macromol Biosci. 2008;8(1):14–24. [Google Scholar] [PubMed]
- 9.Gu J-D. Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegradation. 2003;52(2):69–91. [Google Scholar] [PubMed]
- 10.Sivan A. New perspectives in plastic biodegradation. Curr Opin Biotechnol. 2011;22(3):422–6. [Google Scholar] [PubMed]
- 11.Mayer A. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60(6):551–65. [Google Scholar] [PubMed]
- 12.Priyanka N, Archana T. Biodegradability of Polythene and Plastic by The Help of Microorganism: A Way for Brighter Future. J Environ Anal Toxicol. 2011;01(02). [Google Scholar] [PubMed]
- 13.Rehm Bha. Polyester synthases: natural catalysts for plastics. Biochem J. 2003;376(1):15–33. [Google Scholar] [PubMed]
- 14.Cha D, Ha HS, Lee SK. Metabolic engineering of Pseudomonas putida for the production of various types of short-chain-length polyhydroxyalkanoates from levulinic acid. Bioresour Technol. 2020;309:123332. [Google Scholar] [PubMed]
- 15.Suriyamongkol P, Weselake R, Narine S, Moloney M, Shah S. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants — A review. Biotechnol Adv. 2007;25(2):148–75. [Google Scholar] [PubMed]
- 16.Doi Y. Synthesis and degradation of polyhydroxyalkanoates in Alcaligenes eutrophus. FEMS Microbiol Lett. 1992;103(2– 4):103–8. [Google Scholar] [PubMed]
- 17.Page W. Production of polyhydroxyalkanoates by Azotobacter vinelandii UWD in beet molasses culture. FEMS Microbiol Lett. 1992;103(2–4):149–57. [Google Scholar] [PubMed]
- 18.Valappil SP, Boccaccini AR, Bucke C, Roy I. Polyhydroxyalkanoates in Gram-positive bacteria: insights from the genera Bacillus and Streptomyces. Antonie Van Leeuwenhoek. 2006;91(1):1–17. [Google Scholar] [PubMed]
- 19.Belal EB. Production of Poly-&betaHydroxybutyric Acid (PHB) by Rhizobium elti and Pseudomonas stutzeri. Curr Res J Biol Sci. 2013;5(6):273–84. [Google Scholar] [PubMed]
- 20.Gouda MK, Swellam AE, Omar SH. Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res. 2001;156(3):201–7. [Google Scholar] [PubMed]
- 21.Lee K-M, Gimore DF, Huss MJ. Fungal Degradation of the Bioplastic PHB (Poly-3- hydroxy- butyric acid). J Polym Environ. 2005;13(3):213–9 [Google Scholar] [PubMed]
- 22.RamKumar Pandian S, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, Gurunathan S. Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresour Technol. 2010;101(2):705–11. [Google Scholar] [PubMed]
- 23.RamKumar Pandian S, Deepak V, Kalishwaralal K, Rameshkumar N, Jeyaraj M, Gurunathan S. Optimization and fed-batch production of PHB utilizing dairy waste and sea water as nutrient sources by Bacillus megaterium SRKP-3. Bioresour Technol. 2010;101(2):705–11. [Google Scholar] [PubMed]
- 24.Rahman A, Miller CD. Microalgae as a Source of Bioplastics. In: Algal Green Chemistry. Elsevier; 2017. p. 121–38. [Google Scholar] [PubMed]
- 25.Hempel F, Bozarth AS, Lindenkamp N, Klingl A, Zauner S, Linne U, et al. Microalgae as bioreactors for bioplastic production. Microb Cell Fact. 2011;10(1):81. [Google Scholar] [PubMed]
- 26.Venkata Mohan S, Hemalatha M, Chakraborty D, Chatterjee S, Ranadheer P, Kona R. Algal biorefinery models with self-sustainable closed loop approach: Trends and prospective for bluebioeconomy. Bioresour Technol. 2020; 295:122128. [Google Scholar] [PubMed]
- 27.Jankowska E, Sahu AK, Oleskowicz-Popiel P. Biogas from microalgae: Review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew Sustain Energy Rev. 2017; 75:692–709. [Google Scholar] [PubMed]
- 28.Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: A review. Renew Sustain Energy Rev. 2010 ;14(1):217–32 [Google Scholar] [PubMed]
- 29.Tripathi AD, Srivastava SK, Singh RP. Statistical optimization of physical process variables for bio-plastic (PHB) production by Alcaligenes sp. Biomass and Bioenergy. 2013; 55:243–50 [Google Scholar] [PubMed]
- 30.Yang Y-H, Brigham CJ, Budde CF, Boccazzi P, Willis LB, Hassan MA, et al. Optimization of growth media components for polyhydroxyalkanoate (PHA) production from organic acids by Ralstonia eutropha. Appl Microbiol Biotechnol. 2010;87(6):2037–45. [Google Scholar] [PubMed]
- 31.Aramvash A, Akbari Shahabi Z, Dashti Aghjeh S, Ghafari MD. Statistical physical and nutrient optimization of bioplastic polyhydroxybutyrate production by Cupriavidus necator. Int J Environ Sci Technol. 2015;12(7):2307–16. [Google Scholar] [PubMed]
- 32.Kavitha G, Kurinjimalar C, Sivakumar K, Kaarthik M, Aravind R, Palani P, et al. Optimization of polyhydroxybutyrate production utilizing waste water as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int J Biol Macromol. 2016; 93:534–42. [Google Scholar] [PubMed]
- 33.Umesh M, Mani VM, Thazeem B, Preethi K. Statistical Optimization of Process Parameters for Bioplastic (PHA) Production by Bacillus subtilis NCDC0671 Using Orange Peel-Based Medium. Iran J Sci Technol Trans A Sci. 2018;42(4):1947–55. [Google Scholar] [PubMed]
- 34.Al-Battashi H, Annamalai N, Al-Kindi S, Nair AS, Al-Bahry S, Verma JP, et al. Production of bioplastic (poly-3-hydroxybutyrate) using waste paper as a feedstock: Optimization of enzymatic hydrolysis and fermentation employing Burkholderia sacchari. J Clean Prod. 2019; 214:236–47. [Google Scholar] [PubMed]
- 35.Favaro L, Basaglia M, Casella S. Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: a review. Biofuels, Bioprod Biorefining. 2019;13(1):208–27. [Google Scholar] [PubMed]
- 36.Pries A, Steinbuchel A, Schlegel H. Lactose- and galactose-utilizing strains of poly (hydroxyalkanoic acid)-accumulating Alcaligenes eutrophus and Pseudomonas saccharophila obtained by recombinant DNA technology. Appl Microbiol Biotechnol. 1990;33(4). [Google Scholar] [PubMed]
- 37.Povolo S, Toffano P, Basaglia M, Casella S. Polyhydroxyalkanoates production by engineered Cupriavidus necator from waste material containing lactose. Bioresour Technol. 2010;101(20):7902–7 [Google Scholar] [PubMed]
- 38.Park SJ, Jang Y-A, Lee H, Park A-R, Yang JE, Shin J, et al. Metabolic engineering of Ralstonia eutropha for the biosynthesis of 2-hydroxyacidcontaining polyhydroxyalkanoates. Metab Eng. 2013; 20:20–8. [Google Scholar] [PubMed]
- 39.Park SJ, Jang Y-A, Noh W, Oh YH, Lee H, David Y, et al. Metabolic engineering of Ralstonia eutropha for the production of polyhydroxyalkanoates from sucrose. Biotechnol Bioeng. 2015;112(3):638–43. [Google Scholar] [PubMed]
- 40.Obruca S, Snajdar O, Svoboda Z, Marova I. Application of random mutagenesis to enhance the production of polyhydroxyalkanoates by Cupriavidus necator H16 on waste frying oil. World J Microbiol Biotechnol. 2013;29(12):2417–28. [Google Scholar] [PubMed]
- 41.Riedel SL, Lu J, Stahl U, Brigham CJ. Lipid and fatty acid metabolism in Ralstonia eutropha: relevance for the biotechnological production of value-added products. Appl Microbiol Biotechnol. 2014;98(4):1469–83. [Google Scholar] [PubMed]
- 42.Mifune J, Nakamura S, Fukui T. Engineering of pha operon on Cupriavidus necator chromosome for efficient biosynthesis of poly(3-hydroxybutyrate-co-3- hydroxyhexanoate) from vegetable oil. Polym Degrad Stab. 2010;95(8):1305–12. [Google Scholar] [PubMed]
- 43.Solaiman DKY, Ashby RD, Foglia TA. Production of polyhydroxyalkanoates from intact triacylglycerols by genetically engineered Pseudomonas. Appl Microbiol Biotechnol. 2001;56(5–6):664–9 [Google Scholar] [PubMed]
- 44.Slater SC, Voige WH, Dennis DE. Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol. 1988;170(10):4431–6 [Google Scholar] [PubMed]
- 45.Schubert P, Steinbüchel A, Schlegel HG. Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol. 1988;170(12):5837–47. [Google Scholar] [PubMed]
- 46.Balaji S, Gopi K, Muthuvelan B. A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal Res. 2013;2(3):278–85. [Google Scholar] [PubMed]
- 47.Bohmert-Tatarev K, McAvoy S, Daughtry S, Peoples OP, Snell KD. High Levels of Bioplastic Are Produced in Fertile Transplastomic Tobacco Plants Engineered with a Synthetic Operon for the Production of Polyhydroxybutyrate. Plant Physiol. 2011;155(4):1690–708. [Google Scholar] [PubMed]
- 48.Jongedijk E, Müller S, van Dijk ADJ, Schijlen E, Champagne A, Boutry M, et al. Novel routes towards bioplastics from plants: elucidation of the methylperillate biosynthesis pathway from Salvia dorisiana trichomes. Takahashi H, editor. J Exp Bot. 2020;71(10):3052–65. [Google Scholar] [PubMed]
- 49.Ahmed T, Shahid M, Azeem F, Rasul I, Shah AA, Noman M, et al. Biodegradation of plastics: current scenario and future prospects for environmental safety. Environ Sci Pollut Res. 2018;25(8):7287–98. [Google Scholar] [PubMed]
- 50.Mohanty AK, Misra M, Hinrichsen G. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol Mater Eng. 2000;276– 277(1):1–24. [Google Scholar] [PubMed]
- 51.Gajendiran A, Subramani S, Abraham J. Effect of Aspergillus versicolor strain JASS1 on low density polyethylene degradation. IOP Conf Ser Mater Sci Eng. 2017; 263:022038. [Google Scholar] [PubMed]
- 52.Trivedi P, Hasan A, Akhtar S, Siddiqui MH, Sayeed U and Khan MKA. Role of microbes in degradation of synthetic plastics and manufacture of bioplastics. J Chem Pharm Res. 2016, 8(3):211-216. [Google Scholar] [PubMed]
- 53.Shahi N, Hasan A, Akhtar S, Siddiqui MH, Sayeed U and Khan MKA. Xylanase: a promising enzyme. J Chem Pharm Res. 2016, 8(3), 334- 339. [Google Scholar] [PubMed]
- 54.Adhikari D, Mukai M, Kubota K, Kai T, Kaneko N, Araki KS, et al. Degradation of Bioplastics in Soil and Their Degradation Effects on Environmental Microorganisms. J Agric Chem Environ. 2016;05(01):23–34. [Google Scholar] [PubMed]
- 55.Imam SH, Gordon SH, Shogren RL, Tosteson TR, Govind NS, Greene R V. Degradation of Starch– Poly(β-Hydroxybutyrate-Co-βHydroxyvalerate) Bioplastic in Tropical Coastal Waters. Appl Environ Microbiol. 1999;65(2):431–7. [Google Scholar] [PubMed]
- 56.Emadian SM, Onay TT, Demirel B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017; 59:526–36. [Google Scholar] [PubMed]
- 57.Nishida H, Tokiwa Y. Effects of higher-order structure of poly(3-hydroxybutyrate) on its biodegradation. II. Effects of crystal structure on microbial degradation. J Environ Polym Degrad. 1993;1(1):65–80. [Google Scholar] [PubMed]
- 58.Tsuji H. Hydrolytic Degradation. In: Poly(Lactic Acid). Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2010. p. 343–81. [Google Scholar] [PubMed]
- 59.Linder M. Ripe for disruption: reimagining the role of green chemistry in a circular economy. Green Chem Lett Rev. 2017;10(4):428–35. [Google Scholar] [PubMed]

