Department of Bioengineering, Integral University, Lucknow, 226026, Uttar Pradesh, India
Mesenchymal stem cells (MSCs) are adult stem cells that can be isolated from humans or animals. Human MSCs are multipotent stem cells that have a great potential to differentiate into mesoderm as well as endoderm lineage. Human mesenchymal stem cells (HMSCs) can be cultured for a long time purpose in cell-specific media without any abnormalities. Up to now, MSCs are isolated from different tissues including adipose tissue, amniotic fluid, endometrium, dental tissues, umbilical cord, and Wharton's jelly. MSCs possess low immunogenicity and strong immunomodulation potential; they secrete cytokines and use immune receptor which keeps the check on the microenvironment within the tissue. Their multilineage potential, immunomodulation, and production of anti-inflammatory molecules made MSCs use in clinical research and treatments. Their differential potential together with the property to secrete soluble factors and release extracellular vesicles like exosomes and microvesicles enable them to serve the purpose of tissue repair. Recent studies suggested that extracellular vesicles that serve as the cargo of mRNA, miRNA, and proteins are responsible for the therapeutic effects of MSCs. MSCs are now widely considered as the best tool for regenerating tissues due to their fast self-renewal property, their easy accessibility with a lesser ethical concern for clinical applications. MSCs clinical applications include cartilage and bone repair, tissue healing, heart, and neuronal regeneration, and treatment of different immune disorders including graft versus host disease (GvHD). Their great potential allows them to treat even many incurable diseases. This review gives detailed information on MSCs, their sources, definite properties, biomarkers, and applications. The wide potential of MSCs in today’s scenario is seen in the field of toxicology studies and cancer research.
KeywordsMesenchymal stem cells, cluster of differentiation, immunomodulation, clinical applications, toxicology, and cancer research
The term ‘Stem cell’ originated in the 19th century in context to two major embryological questions of that time i.e. the origin of the hematopoietic system and the continuity of germline. The term ‘Mesenchymal stem cell’ was given by Caplan in the year 1991. Mesenchymal stem cells (MSCs) are multipotent stem cells with a capacity to differentiate into the different variety of cells which can be detached and can be cultured easily with in vitro conditions. These are non-hematopoietic stromal cells that contribute to the regeneration of mesenchymal tissues. Adipose, tendon, ligament, muscle, bone cartilage are examples of the mesenchymal tissues that can be regenerated using MSCs. They must express CD105, CD90, and CD73 and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR surface molecules.[1] MSCs were first observed nearly 140 years ago by German pathologist Julius Friedrich Cohnheim. Later Alexander Friedstein and colleagues placed the complete bone marrow in a plastic dish, on removing non-adherent cells after 4 hours. They got adherent cells that were heterogeneous in appearance and were strongly adhered to surface posses spindle shape, form foci with 2 or 4 cells, which began to multiply simultaneously after 2-4 days.[2] On passaging regularly cells turned homogeneously fibroblastic. They got observations of cells differentiating into clumps (cologenic colonies, colony-forming units-fibroblast) that are similar to minute deposits of cartilage and bones. Mesenchymal stem cells have the potential to interact with components of both adaptive and innate immunity which enables them to modulate various effecter molecules. These bioactive molecules can suppress the local immune system, apoptosis, enhance angiogenesis, inhibit fibrosis and enable the mitosis as well as differentiation of stem cells.[3] MSCs are devoid of allogeneic rejection in animal as well as human models; this is proved with several in vitro studies. This property of MSCs is due to its three incredibly significant properties. Firstly, MSCs lack MHCII and other co-stimulatory molecules expression thus they are hypoimmunogenic. Secondly, MSCs can prevent T-cell response directly by disrupting Natural Killer cells and CD8+ and CD4+ cells and their function or by indirectly with modulation of dendritic cells.[4] Thirdly, MSCs create a local suppressive microenvironment with the production of prostaglandins and interleukins-10 and with an expression of indoleamine 2, 3-dioxygenase. MSCs are referred to as highly regulated and self-renewing populations with the obvious potential in avoiding allogeneic rejection.[5]
Mesenchymal Stem Cells are isolated from different sources for various purposes. The bone marrow is considered the major source of MSCs in humans and animals. MSCs from fetal origin have a higher proliferation rate and a high number of Vitro passages until senescence. MSCs from adipose tissue and bone marrow have great potential to create a high quantity of CFU-F colonies; this determines their high degree of stemness. Studies suggested that properties of Mesenchymal Stem Cells from bone marrow are associated with the donor’s age.[5]
Stem cell markers are the genes and their products used for isolation as well as identification of stem cells. Stem cells are categorized into different types based on the presence of markers that have a particular significance. On the surface of every cell, there are specialized proteins, referred to as receptors which are capable of binding to molecules in a way to enable communication between cells to carry different specific functions in the body. These receptors are named Stem Cell markers. Stem cell markers are used as tags or to mark cells which enable them to be separated and isolated easily. Identification of cell surface markers is done with various methods like using fluorescent tags and visualizing with a microscope, using advanced techniques like fluorescence-activated cell sorting (FACS).[6]
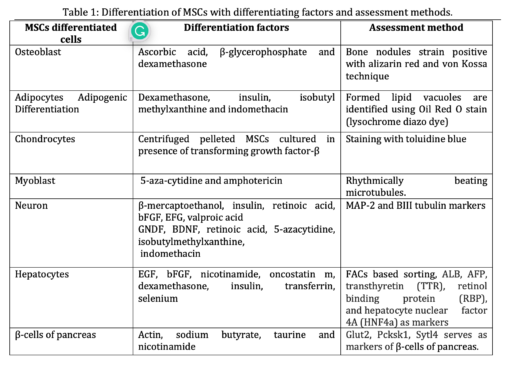
The minimum criteria used to identify MSCs involve:(a) They are plastic adherent under in-vitro culture conditions. (b) Expresses CD105, CD73, and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA‐DR. other include CD13, CD29, CD44, and CD10 (c) differentiate into osteoblasts, adipocytes, and chondrocytes in vitro culture conditions. MSCs possess various biomarkers, these are proteins that enable to identify and isolate MSCs (Table 1).
Stro-1
Stro-1(75 kD) is a type 1 protein that translocates to the cell membrane from the endoplasmic reticulum due to the depletion of intracellular calcium concentration. Stro‐1 is expressed in the dental‐synovial membrane, decidua parietalis‐derived MSCs and multipotent dermal fibroblasts, Umbilical cord blood, Umbilical cord, peripheral blood‐derived MSCs are negative/low for Stro‐1 expression. Stro-1 is not expressed in all types of MSCs.[6] The expression of Stro-1 bone marrow MSCs increases with culture time.
CD271
CD271 is not universally expressed in different MSCs. It is majorly expressed in high proportion in the bone marrow and adipose tissue and is too expressed in person ligaments of MSCs, whereas CD271 is expressed with a low level in placenta-derived MSCs and it is not expressed in the synovial membrane, peripheral blood, Umbilical cord, and Umbilical cord blood MSCs. The role of CD271 is in maintaining clonogenicity and functions of MSCs. CD271 is used for the selection and identification of CFU-Fs from Bone marrow mononuclear cells.CD271 is a neurotrophin receptor that stimulates neuronal cells in survival and differentiation.[7]
SSEA‐4
SSEA‐4 (Stage-Specific Embryonic Antigen 4) an embryonic stem cell marker used in isolation of MSCs from bone marrow, its expression grows over some time in bone marrow culture. SSEA -4(+) cells expand easily and too show tripotency while SSEA-4(-) cells fail to differentiate and grow.[8]
CD146
CD146 (Cluster of Differentiation 146), 113kDa also name as melanoma cell adhesion molecule (MCAM). CD146 is expressed almost in all sources of MSCs not only in bone marrow only. CD146 has a greater CFU‐Fs enrichment capacity than other markers of MSCs like CD90, Stro‐1, or CD133. CD146 is expressed in MSCs with higher multipotency, the MSCs with CD146 have the high supporting capacity for hematopoiesis.[9]
CD49f
CD49f (ITGA6 gene), also known as Integrin alpha-6 regulates a variety of signaling pathways. Oct-4 and Sox-2 are responsible for the expression of CD49f, with the knockdown of CD49f in Embryonic Stem Cells, they differentiate into three germ layers, suggesting that CD49f is involved in maintaining pluripotency of ESCs. CD49f is not a specific marker of MSCs also expressed in a variety of other cells (endothelial cells, monocytes, platelets, and thymocytes).[10]
CD349
CD349, transmembrane‐spanning receptor, which is activated by Wnt ligands, they are expressed in periodontal ligament-derived MSCs. In CD349(+) cells CFU‐F was enriched 60‐fold. CD349(−) cells proliferate at a higher rate than CD349(+) cells. It is not regarded as a critical MSCs marker or essential in MSCs functioning.[11]
GD2
GD2 is a single surface marker sufficient to isolate MSCs from BM, it is the neural ganglioside. GD2 is highly expressed in CD45(−) CD73(+) but not expressed in CD45(−) BM cells. It is highly expressed in adipose tissue MSCs and umbilical cord MSCs.[12] Some CD34(+) or CD19(+) bone marrow cells express GD2 expression.[11]
SSEA‐3
Stage‐specific embryonic antigen‐3 (SSEA‐3) is a pluripotent embryonic stem cell marker. SSEA‐3 is a glycosphingolipid (an oligosaccharide). SSEA‐3(+) cells in MSCs differentiate into ectodermic, endodermal, and mesodermal lineage cells in vivo.[12]
Mesenchymal stem cells are generally collected using a density gradient centrifuge and require 10% serum (FBS) to culture mononuclear adherent cells in in vitro conditions. The sites of MSCs isolation are bone marrow(mainly), adipose tissue, amniotic fluid, periosteum, fetal tissue, etc.[13] MSCs are cultured in deprivation media to eliminate contamination. The culture remains morphologically heterogeneous having narrow spindle-shaped cells, large polygonal cells, and some cuboidal cells in confluent culture. Several reports suggest the separation and isolation of MSCs with a negative selection process where cells of hematopoietic lineage are removed and the other process includes the use of antibodies as the process of positive selection of MSCs. Le Blanc and colleagues suggested that MHC antigen expression changes from fetal state to adult human being. Adult MSCs are with an intermediate expression of Class I Major Histocompatibility Complex (MHC) with the absence of human leukocyte antigen class II (HLA class II) whereas the fetal liver-derived Human Mesenchymal stem cells (hMSCs) lack MSCs class II.[14] Another way to identify MSCs is based on their differentiation potential i.e. the differentiation of MSCs into, adipose tissue, cartilage, myoblast, and neuron-like cells.[15],[16] MSCs with fetal origin possess a faster rate of proliferation than with adult human isolated MSCs. MSCs derived from bone marrow have a high degree of stemness and have a high potential to differentiate into osteoblast whereas MSCs from umbilical cord blood lack the tendency to differentiate into adipocytes.[17]
Cultured MSCs exhibit the property of immunomodulation. MSCs possess the property to interact as well as regulate the functions of cells responsible for immune responses as shown in figure 1. They do so by various means like by hindering apoptosis of activated and naive neutrophils, reducing the number of neutrophils binding the vascular endothelial cells, inhibiting the complement-mediated effects of peripheral blood mononuclear cell proliferation, cytokines from activated MSCs enable neutrophil chemotaxis, and also secretion of chemokines that are proinflammatory that regulate phagocytic macrophage properties.[18]
The functions of NK cells, B cells, and memory T cells are suppressed as well. The MSCs migrate to the damaged renal tissue and secrete several cytokines and chemokines that can correct the course of injury.[19] MSCs release: vascular endothelial growth factor, stromal cell-derived factor-1, fibroblast growth factor, insulin-like growth factor (IGF), keratinocyte growth factor, hepatocyte growth factor, and monocyte chemoattractant protein-1 for endogenous cell repair activation. MSCs microvesicles maintain the ATP supply after injury with mitochondria transfer. MSCs can block the differentiation of CD34+ cells by direct contact or by secreted paracrine factors and can block their differentiation into mature dendrite cells.[20]

Figure 1: Immunomodulatory properties of Mesenchymal Stem Cells.
The property of paracrine action was observed by Haynes worth et. al, observed MSCs release some factors or mediators that regulate various functions i.e. increase angiogenesis, reduce apoptosis and fibrosis, enhance neuronal survival and differentiation, stimulate extracellular matrix remodeling, restrict local inflammation, and adjust immune responses. These mediators through paracrine action enable the cell or adjacent cells to release active factors.[21]
Extracellular vesicles (EVs), which contain many physiologically active substances such as nucleic acids and proteins, are one of the MSC-based therapies. The contents of EVs are transported across cells for intercellular communication, which leads to the regulation of recipient cell homeostasis. Because EVs can remain therapeutically effective cargo of parent cells and are free of numerous ethical problems in cell-based therapies, they represent a significant way of cell-free therapies such as tissue repair or regeneration. The creation of new EV-dependent medicines is difficult due to a lack of standard protocols in EV extraction processes and their pharmacological characteristics and mechanisms. EVs are possible biomarkers for treating neuronal disorders including Alzheimer’s and Parkinson’s.[21] (Table 2).

In the process of drug development, clinical and pre-clinical trials are a must to ensure human safety towards the drugs and their consequent doses. The pre-clinical trials were traditionally performed on animals which serve as a loss to animal life, time, and a growing issue towards ethical issues. The pre-clinical studies are to determine the mutagenicity, acute, sub-chronic, and chronic toxicity, developmental and reproductive toxicity, carcinogenicity, and safety pharmacology. The pre-clinical testing is done with different protocols underlying Good Laboratory Practice regulation. A study including Draize in vivo rabbit irritation test (OECD, 2002), the toxicant was applied on unanesthetized rat’s skin and eyes, which involve painful and invasive procedures that are harmful to animal welfare throughout the world to overcome the issue alternative i.e. (non-animal) methods for toxicity testing using 3R principle (Replacement, Reduction, and Refinement).For the purpose stem cells and stem cell-derived specific cells to serve for purpose of toxicity testing that includes developmental toxicity, genotoxicity, and tissue-specific toxicity.[22] The unique characteristics of stem cells i.e. self-renewal, infinite proliferation, differentiation into multiple lineages, the stem cell-based approach have proven a valuable tool to enhance the prediction of toxicology providing better information on toxicity effects and risks associated with it in humans without inter-species variability. The stem cell approach with induced pluripotent stem cells is advantageous in personalized toxicity assessment this serves as a better option as in vitro model system in comparison of immortalized cells (abnormal phenotype ) and primary cells (small in quantity and have a batch to batch variation).[23]
A study conducted for testing the neuronal toxicity) of Fe3O4NPs (magnetite-nanoparticles with in vitro differentiation of human umbilical cord lining derived mesenchymal stem cells (hCL-MSCs) into neuronal-like cells. These differentiated neuronal cells have the neuron-like phenotype, morphology posses, neuronal-specific markers at different maturation stages, and too possess synaptic markers. In presence of Fe3O4NPs (magnetite-nanoparticles) the neuron-like cells (hNLCs) gets affected, concentration- and time-dependent reduction of hNLCs viability is observed (using trypan blue dye, ATP uptake ),cell density decrease with 20-50% and apoptotic effects were detected at >/=10mug/ml. In three-day differentiated hNLCs toxicity appeared early and remains up to 48 hrs than those cells with 8-day differentiation. This study concluded that hCL-MSCs are easy to differentiate into neuron-like cells that are susceptible to Fe3O4NPs and are used as a new in vitro model for the NP evaluation.[24]
A study was conducted to determine the effects of lead (Pb) exposure on Adipose-derived stem cells (ADSCs) differentiation in neuronal media. The third passage ADSCs differentiated into a neural cell with differentiation media in 16 days. The ADSCs were exposed to lead before and with differentiation on days 1,7 and 14. The cell’s viability was determined in the process. They concluded that undifferentiated ADSCs viability is unaffected by the lead exposure and cells were more vulnerable to lead exposure with early stage of differentiation and with different stages of differentiation lead exposure affects gene expressions. ADSCs are used as an alternative method for determining the neurodevelopment toxicity potential of chemicals.[25]
A recent study suggested that chemotherapy-induced peripheral neuropathy (CIPN) is caused due to Chemotherapeutic drugs exposure’s side effects disrupting the nervous system. Evidence determined that MSCs use enable the reduction of ROS formation, reduce the level of inflammation and apoptosis, and enable axon regeneration after nerve damage. Mesenchymal stem cell-based therapies can serve as a cure for patients suffering from CIPN.[26]
Mesenchymal stem cells are serving as a promising source in personalized cell-based therapies.[27] MSCs tumor tropic property is preferentially used in targeting cancer cells. MSCs are genetically modified and engineered to play the role of anticancer agents, [28] which enable the different processes of tumor formation by blocking the pathway that suppresses both apoptosis and proliferation. However, some studies demonstrated that MSCs can promote tumor progression and metastasis by influencing signaling pathways.[29]
MSCs derived from a large number of sources can be isolated easily and expanded with low immunogenicity. MSCs transplantation is a better option than allogeneic hematopoietic stem cell transplantation it is so because after allogeneic hematopoietic stem cell transplantation there are chances of development of graft-versus-host diseases (GvHD) and autoimmune diseases in the recipient which is a serious compilation and affects the transplantation efficacy. MSCs transplantation is thus a better option with low immunogenicity, its immunosuppressive effects, and immunoregulatory functions.[30]
The MSCs possess both pros as well as anti-oncogenic properties. A study suggested genetically engineered Mesenchymal stem cells and nanoparticles are employed to treat the tumor and improve the antitumor properties of these cells. The MSCs ability i.e. tumor-homing ability and exomes to tumor niche act as a promising tool in targeted delivery of therapeutic agents to the site of tumor progression and development. Studies investigated Mesenchymal Stem cells specifications; their pro and anti-oncogenic properties in treating breast cancer and serve as a potent cellular vehicle in the targeted breast cancer therapy.[31]
MSCs have the potential to differentiate into a diverse range of cell types and secrete regenerative factors which serve for purpose of repair to damaged tissues. Wharton’s jelly-derived MSCs (WJ-MSCs) do not express MHC-II, therefore, these exhibit immunomodulatory properties, this makes them be a good alternative for allogeneic as well as xenogeneic transplantations in cellular therapies.[32] On tissue damage, MSCs move from bone marrow and areas near blood vessels within a tissue into the blood continuously circulating and finally accumulating at the damage site. That is why MSCs transplantation therapies are nowadays an interesting area of scientists for the treatment of various intractable diseases in humans. A study determined that some components from the plant as their source can activate cell functions. Compounds like phosphatidylinositol 3-kinase and mitogen-activated protein kinase (MAPK) activate cell signaling pathways, consequently, promoting cell migration of MSCs. Also, these plant-derived components promoted the recruitment of MSCs to damage the site or tissue (homing property) and later enhance healing.[33]
A recent study determined how the exomes from MSCs exhibit functions similar to MSCs. The study determined a novel and viable cell-free therapeutic strategy by UC-MSC-derived exosomes in treating endometrial traumas that may cause an intrauterine adhesion (IUAs) which leads to infertility. A construct was designed of exomes of MSCs and a scaffold made of collagen, for endometrial regeneration in rat endometrial regeneration model, and further investigation was done on regeneration mechanism using macrophage immunomodulation. The transplanted collagen scaffold together with exome in a model of rat shows a positive response towards the regeneration of endometrium. The transplantation too induced the potential of collagen remodeling, increased the expression of estrogen receptor alpha/progesterone receptor, and too restored fertility in the studied rat model. Immunomodulatory functions of miRNAs of UC-MSCs enabled fertility restoration by endometrium regeneration with the treatment of collagen scaffold and exomes of UC-MSCs.[34]
MSCs are having promising potential in regenerative medicines as well as in tissue engineering. Studies suggested that spheroid formation with MSCs possess improved stemness maintenance and therapeutic potential when compared with monolayer culture. Dissociated MSCs in TeSR-E8 medium induced formation of the self-assembled spheroid in a conventional tissue culture dish of polystyrene.[35] This Adipose-derived stem cell (ADSC) spheroid enhanced the proliferation and osteogenic potential of ADSC compared to monolayer culture. When reseeded in a serum-containing medium, the stemness biomarker expression level increased than its level present in monolayer culture. Spheroid ADSCs promoted M2 polarization of macrophage both in vitro and in vivo conditions. Patients with acute liver injury and end-stage chronic liver disease are treated with orthotopic liver or hepatocyte transplantation; these therapies are less used due to organ donation shortage. MSCs differentiated into hepatocyte-like cells (HLCs) for the treatment of liver diseases are expected to overcome the shortage of organ donors. A study determined that rhesus macaque BM-MSCs and AT-MSCs had hepatogenic differentiation ability and was evaluated by morphology, glycogen accumulation, urea synthesis, and hepatocyte marker gene expression. The study suggests that MSCs from bone marrow are transdifferentiated into hepatocyte-like cells (HLCs) for acute or chronic liver injuries in animals as well as in humans.[36]
Inadequate blood supply and oxygen deficiency to the retina are major causes of retinal detachment and cause multiple pathological events. Death and degeneration of photoreceptor cells are leads to a decline in vision. A study was conducted to investigate the therapeutic effects of exomes derived from MSCs (MSC-Exos) in the retinal detachment model of a male Sprague-Dawley rat. The model developed with subretinal injection of 1% hyaluronic acid in male rats and MSC-Exos were subretinally injected on retinal separation time to study their therapeutic effects of MSC-Exos. The effects were analyzed with RT-PCR, Western blotting, TUNEL assays, and proteomic analysis. Treatment with MSC-Exos maintained normal retinal structure and suppressed photoreceptor cell apoptosis compared with control groups. The proteomic analysis revealed that MSC-Exos possess proteins that have anti-inflammatory, neuroprotective, and anti-apoptotic properties. The study concludes that MSC-Exos have therapeutic effects on retinal detachment and are used in treating patients with photoreceptor cell degeneration.[37]
Since the last three decades research on MSCs has made continuous progress. There seems a wide potential of hMSCs for clinical purposes without any side effects reported. There are more than 1138 registered clinical trials listed and the number is continuously increasing. The therapeutic potential of MSCs makes them a promising source of treatment for various diseases and disorders. The characteristic of MSCs to differentiate into different cell lineage and the absence of ethical issues related to its use makes them a suitable choice in cell therapy.
Conflicts of Interest
The author declares that there are no conflicts of interest relevant to this article.
References
- 1.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7. [Google Scholar] [PubMed]
- 2.Baghban Eslaminejad M. Mesenchymal stem cells: history, isolation and biology. J Iran Anatomic sci. 2007;5(18):49-59. [Google Scholar] [PubMed]
- 3.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends in molecular medicine. 2012;18(2):128-34. [Google Scholar] [PubMed]
- 4.Rasmusson I. Immune modulation by mesenchymal stem cells. Experimental cell research. 2006;312(12):2169-79. [Google Scholar] [PubMed]
- 5.English K, Mahon BP. Allogeneic mesenchymal stem cells: agents of immune modulation. Journal of cellular biochemistry. 2011;112(8):1963-8. [Google Scholar] [PubMed]
- 6.Ning H, Lin G, Lue TF, Lin C-S. Mesenchymal stem cell marker Stro-1 is a 75kd endothelial antigen. Biochemical and biophysical research communications. 2011;413(2):353-7. [Google Scholar] [PubMed]
- 7.Álvarez-Viejo M, Menéndez-Menéndez Y, Otero-Hernández J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World journal of stem cells. 2015;7(2):470. [Google Scholar] [PubMed]
- 8.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743-51. [Google Scholar] [PubMed]
- 9.Espagnolle N, Guilloton F, Deschaseaux F, Gadelorge M, Sensébé L, Bourin P. CD 146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. Journal of cellular and molecular medicine. 2014;18(1):104-14. [Google Scholar] [PubMed]
- 10.Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, et al. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem cells. 2012;30(5):876-87. [Google Scholar] [PubMed]
- 11.Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007 Jun;1106:262-71. [Google Scholar] [PubMed]
- 12.Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007 May 15;109(10):4245-8. [Google Scholar] [PubMed]
- 13.Vats A, Tolley N, Polak J, Buttery L. Stem cells: sources and applications. Clinical Otolaryngology & Allied Sciences. 2002;27(4):227-32. [Google Scholar] [PubMed]
- 14.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007;25(11):2739-49. [Google Scholar] [PubMed]
- 15.Yoneno K, Ohno S, Tanimoto K, Honda K, Tanaka N, Doi T, et al. Multidifferentiation potential of mesenchymal stem cells in three‐dimensional collagen gel cultures. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials. 2005;75(3):733-41. [Google Scholar] [PubMed]
- 16.Bajetto A, Thellung S, Dellacasagrande I, Pagano A, Barbieri F, Florio T. Cross talk between mesenchymal and glioblastoma stem cells: Communication beyond controversies. Stem Cells Transl Med. 2020 Nov;9(11):1310-1330. [Google Scholar] [PubMed]
- 17.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental hematology. 2005;33(11):1402-16. [Google Scholar] [PubMed]
- 18.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature Reviews Immunology. 2012;12(5):383-96. [Google Scholar] [PubMed]
- 19.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clinical & Experimental Immunology. 2011;164(1):1-8. [Google Scholar] [PubMed]
- 20.Wu Y, Zhao RC, Tredget EE. Concise review: bone marrow‐derived stem/progenitor cells in cutaneous repair and regeneration. Stem cells. 2010;28(5):905-15. [Google Scholar] [PubMed]
- 21.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006;98(5):1076-84. [Google Scholar] [PubMed]
- 22.Krewski D, Andersen ME, Tyshenko MG, Krishnan K, Hartung T, Boekelheide K, et al. Toxicity testing in the 21st century: progress in the past decade and future perspectives. Archives of toxicology. 2020;94(1):1-58. [Google Scholar] [PubMed]
- 23.Scott CW, Peters MF, Dragan YP. Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicology letters. 2013;219(1):49-58. [Google Scholar] [PubMed]
- 24.De Simone U, Spinillo A, Caloni F, Gribaldo L, Coccini T. Neuron-like cells generated from human umbilical cord lining-derived Mesenchymal stem cells as a new in vitro model for neuronal toxicity screening: Using magnetite nanoparticles as an example. International journal of molecular sciences. 2020;21(1):271. [Google Scholar] [PubMed]
- 25.Qasemian Lemraski M, Soodi M, Fakhr Taha M, Zarei MH, Jafarzade E. Study of lead-induced neurotoxicity in neural cells differentiated from adipose tissue-derived stem cells. Toxicology mechanisms and methods. 2015;25(2):128-35. [Google Scholar] [PubMed]
- 26.Boukelmoune N. Nasal Mesenchymal Stem Cell Treatment for the Repair of Chemotherapy-Induced Neurotoxicities: Let the Trojan Horse In!: University Utrecht; 2020. [Google Scholar] [PubMed]
- 27.Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem cell research & therapy. 2011;2(2):14. [Google Scholar] [PubMed]
- 28.Uchibori R, Tsukahara T, Ohmine K, Ozawa K. Cancer gene therapy using mesenchymal stem cells. International journal of hematology. 2014;99(4):377-82. [Google Scholar] [PubMed]
- 29.Shojaei S, Hashemi SM, Ghanbarian H, Salehi M, Mohammadi‐Yeganeh S. Effect of mesenchymal stem cells‐derived exosomes on tumor microenvironment: Tumor progression versus tumor suppression. Journal of cellular physiology. 2019;234(4):3394-409. [Google Scholar] [PubMed]
- 30.Toubai T, Paczesny S, Shono Y, Tanaka J, Lowler KP, Malter CT, et al. Mesenchymal stem cells for treatment and prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation. Current stem cell research & therapy. 2009;4(4):252-9. [Google Scholar] [PubMed]
- 31.Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of mesenchymal stem cells for therapeutic agent delivery in anti-tumor treatment. Frontiers in pharmacology. 2018;9:259. [Google Scholar] [PubMed]
- 32.Abbaszadeh H, Ghorbani F, Derakhshani M, Movassaghpour AA, Yousefi M, Talebi M, Shamsasenjan K. Regenerative potential of Wharton's jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. J Cell Physiol. 2020;235(12):9230-9240. [Google Scholar] [PubMed]
- 33.Maeda A. Recruitment of Mesenchymal Stem Cells to Damaged Sites by Plant-Derived Components. Front Cell Dev Biol. 2020; 9;8:437. [Google Scholar] [PubMed]
- 34.Xin L, Lin X, Zhou F, Li C, Wang X, Yu H, et al. A scaffold laden with mesenchymal stem cell-derived exosomes for promoting endometrium regeneration and fertility restoration through macrophage immunomodulation. Acta biomaterialia. 2020;113:252-66. [Google Scholar] [PubMed]
- 35.Zhao Y, Xiao E, Lv W, Dong X, He L, Wang Y, Zhang Y. A Chemically Defined Serum-Free Culture System for Spontaneous Human Mesenchymal Stem Cell Spheroid Formation. Stem Cells Int. 2020 Mar 7;2020:1031985. [Google Scholar] [PubMed]
- 36.Wang J, Fu X, Yan Y, Li S, Duan Y, Inglis BM, et al. In vitro differentiation of rhesus macaque bone marrow‑and adipose tissue‑derived MSCs into hepatocyte‑like cells. Experimental and therapeutic medicine. 2020;20(1):251-60. [Google Scholar] [PubMed]
- 37.Ma M, Li B, Zhang M, Zhou L, Yang F, Ma F, et al. Therapeutic effects of mesenchymal stem cell-derived exosomes on retinal detachment. Experimental eye research. 2020;191:107899. [Google Scholar] [PubMed]