*Corresponding author at: Department of Biotechnology, Delhi Technical University, Delhi-110042, India smitar@dtu.ac.in https://orcid.org/0000-0002-3812-5314 https://doi.org/10.37881/1.637
There has been an increase in the incidence of diseases and injuries which has encouraged the advancements in treatments that could repair tissues as well as minimize the dependence on organ transplantation and tissue loss. Regenerative medicine based on stem cells is a newer integrative domain that has the potential to facilitate the regeneration of diseased and damaged tissues and organs. Stem cells can be effectively exploited for medical purposes with no severe challenges, according to a wide range of literature as well as long-term human and animal studies conducted on them. Amongst several types of stem cells, placenta-derived mesenchymal stem cells possess unique immunomodulatory characteristics as well as the potential to differentiate into various cell types, which make them suitable candidates for cellular therapies for many chronic diseases including cancers, heart and liver disorders, ulcers, bone damage, and neurological diseases, etc. The present study thus aims at providing a comprehensive overview of placenta-derived mesenchymal stem cells, their types, and potentially therapeutic for various chronic and non-chronic diseases. Recent pre-clinical and clinical studies conducted on them have also been incorporated.
KeywordsPlacental mesenchymal stem cell (PMSC), cell therapy, clinical trials, regenerative medicine, multipotent.
In recent times, modern science and medicine have seen an appreciable rise in stem cell research which has garnered recognition globally attributed to its enormous therapeutic potential in the field of regenerative medicine.[1] This is a result of the regenerative capability as well as the pliability possessed by the stem cells. These stem cells manifesting therapeutic potential originate and develop from embryonic as well as adult tissues. Nonetheless, ethical concerns along with the challenges in procuring them and tumorigenicity limit the clinical usage of embryonic stem cells. The adult stem cells have been discovered in various tissues and organs, such as bone marrow[2], brain, placenta[3], skin, adipose tissue le, umbilical cord[4], etc. However, the amount of stem cells obtained from adult tissues is limited. There are numerous risks involved in isolating them and these cells possess a minimal range of differentiation and proliferation after their extraction from the body. As a result, it is burdensome to obtain large quantities of stem cells from them. On the other hand, a good source of mesenchymal stem cells (MSCs) in the human placenta, a vital part of the body found at the time of pregnancy for assisting fetal development. It is a multicellular immunoregulatory organ where both maternal, as well as fetal cells, exist side-by-side. It performs several functions including exchange of metabolites and regulation of the secretion of hormones between the mother and fetus along with the maintenance of immune tolerance between the two.[5] Placenta is also an abundant reservoir of stem cells in comparison to other sources like bone marrow, adipose tissue, etc. where there is a decline in the recuperation of cells with benefactor’s age.[6] MSCs procured from the placenta reveal various features making them a better alternative for cellular therapy in comparison to their counterparts from adult tissues. The placenta is contemplated to be a medical waste having no ethical issues in its utilization as seen in the case of embryonic stem cells.[3] It is voluntarily removed at the time of birth without the requirement of any encroaching procedures unlike those needed in other adult stem cell sources. Because of their primordial origin, placental cells possess great ambidexterity as well as the ability to differentiate.[7] Stem cells procured from the placenta present a distinctive range of mesenchymal markers,[8],[9] along with the potential to differentiate into neurogenic and glial cells[10], insulin-producing cells[11] as well as liver cells.[12] MSCs acquired from the placenta have been employed for treating a wide range of diseases, including cancer, cardiovascular, neurological, and liver diseases, ulcers, as well as bone and cartilage diseases. These MSCs are of comparatively recent types, exhibiting particular immunomodulatory functions with undetermined mechanisms. Certain soluble factors secreted by placental MSCs appear to be the primary cause for therapeutic effects. This shows that placenta-derived MSCs possess paracrine effects.[13] Contrastingly, because of their migration capabilities and tropism for wounded regions, placental MSCs can also act as mobile carriers for drugs. Recently, there has been immense progress in the field of nanotechnology for curing damaged tissues or organs. Owing to the unique features manifested by placental MSCs, these cells together with nanotechnology will turn out to be a considerable and reassuring domain that may bestow remarkable contributions in the field of stem cell therapy in subsequent times. Therefore, this review seeks to incorporate the formation of placenta-derived mesenchymal stem cells and gives a comprehensive overview of their potential therapeutic applications. Furthermore, the prospects have also been discussed.
MSCs may be extracted from a variety of tissues, including bone marrow, adipose tissue, and synovium, as well as human umbilical cord blood, and bone marrow is one of the most important sources of MSCs.[14] The human body contains adipose tissue (AT) in numerous places, including visceral and subcutaneous fat pads, as well as the pelvic region. AT-derived MSCs, also known as Adipose Stem Cells (ASCs), have comparable properties to Bone marrow-MSCs but, unlike Bone marrow mesenchymal stem cells (BM- MSCs), are easier to isolate in large quantities and have a larger expansile property. The physician must consider the difficulties of obtaining the samples as well as the potential negative consequences of harvesting the cells on the donor while choosing an appropriate cell source. Collecting MSCs from BM- MSCs, for example, might cause pain, hemorrhage, or infection, making it more difficult than harvesting cells from peripheral blood or surgical leftovers like AT or birth-derived tissues.[15] MSCs from peripheral blood cells can be obtained by density gradient centrifugation and a large volume of MSCs can be extracted as compared to bone marrow.[16] However, placental mesenchymal stem cells are harvested with minimal invasiveness thereby being the best candidate for therapeutics.[17]
Mesenchymal stem cells are derived from mesoderm.[18] Specifically, placental mesenchymal stem cells can be derived from amniotic membrane, amniotic fluid, chorionic plate as well as chorionic villus.[19] The amniotic membrane is a placental component that develops in extra-embryonic tissue and provides additional nutrition to the fetus throughout pregnancy.[20] Cells derived from amniotic membranes are called amniotic membrane-derived MSCs (AMe-MSC). They are pluripotent. AMe-MSCs have been shown to reduce inflammation and improve immunomodulation. They induce remyelination, increase angiogenesis, reduce oxidative stress, and control matrix metalloproteinases.[21] Amniotic fluid-derived mesenchymal stem cells (AF-MSCs) are made up of differentiated and undifferentiated progenitor cells generated from fetal tissue. AF-MSCs are more pluripotent and less differentiated than BM-MSCs, having a longer replicative lifetime and proliferative capacity. In AF-MSCs, cytokines and chemokines stimulate vasculogenesis, angiogenesis, and osteogenesis due to their paracrine nature. In vivo, AF-MSCs do not produce teratoma.[22] Because of the ease of their acquisition, multipotency, low immune response, reduced donor damage, and acceptable ethical issue, AM-MSCs and AF-MSCs have been reported as a better new prospective field of regenerative medicine when compared to other MSC sources like chorionic, Wharton’s jelly, etc.[23] The amnion, extra-amniotic mesenchymal stem cells, cytotrophoblast, and syncytiotrophoblast make up the chorionic plate. Chorionic plate-derived MSCs (CP-MSCs) are extracted from chorionic placental plates. Compared to other placental stem cells, CP-MSCs have a greater ability to suppress T-cell proliferation and have a stronger angiogenic potential. Higher genes for differentiation into adipogenic, osteogenic, and hepatogenic lineages are expressed in them.[24] Chorionic villus cells, which are made up of stromal fibroblasts, endothelial cells, and macrophages, have characteristics similar to MSCs.[25] Chorionic villous-derived MSCs (CV-MSCs) are more pluripotent and have a slower aging phenotype than bone marrow-derived stem cells.[26]
Stem cell therapy, also known as regenerative medicine, emphasizes the repair and rejuvenation of injured tissues and organs which may occur due to age, disease, or any physical damage. Such approaches turn out to be beneficial in cases where biological processes are incompetent for responding during the emergence of acute as well as chronic disorders. They may necessitate the transplantation of stem cells which will restore the injured tissues, instigate body functions for repairing of tissues, or work as mobile carriers for delivering remedial agents such as genes, medications, or cytokines. Stem cells are considered exceptional in regenerative medicine because of their ability to restore as well as to differentiate to various specialized cells. Advancements in the field of regenerative medicine have drawn attention to the complex characteristics and heterogeneity of stem cells procured from distinct sources. In the past few years, researchers have traversed various properties of adult MSCs suggesting them to be advantageous in regenerative medicine.[27] Hence, MSCs obtained from several sources have displayed enormous prospects in damaged tissues as well as organ repairing and restoration. Being an immune-privileged organ, placental cells exhibit low immunogenicity in vitro[28] as well as in vivo[29] when xenotransplanted in animals displaying a normal immune response. Researchers have illustrated the viability of placenta- derived cells for allogeneic transplantations.[30] In stem cell therapy, along with tissue repairing, the short-lived paracrine actions are also affected by stem cells. The factors generated and released by stem cells induce these paracrine actions. These factors are responsible for controlling injuries, modulating the immune responses as well as promoting self- restoration in damaged tissues which are still alive.[31] Concerning modulation of the immune system, stem cells obtained from the placenta possess an auxiliary advantage over those derived from other sources. This can be well explained by the primary role of the placenta in fetomaternal immune tolerance.[13] During pregnancy, fetal and, particularly, the tissues of the placenta are responsible for maternal tolerance. They provide immune-privileged conditions and regulate the immune system. MHC class II antigens which generally arbitrate transplant rejection are absent in placental cells.[32] Placental cells express fewer amounts of extremely diverse types of antigens belonging to MHC class I as well as the unconventional type HLA-G (histocompatibility antigen class I, G) which can be responsible for suppressing body defenses along with contributing to immune tolerance during pregnancy.[33],[34] Additionally, placenta-derived cells diverge the maternal immune response towards immune tolerance via the secretion of soluble forms of MHC antigens, hormones, and cytokines.[35],[36] Furthermore, B-cells as well as most of the T-cells vanish leaving behind regulatory T-cells, also called Tregs, as the larger population of T-cells that possess immunosuppressive as well as anti-inflammatory effects.[37]
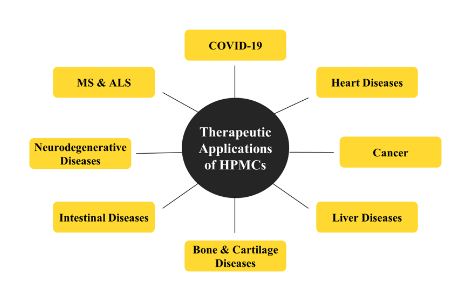
Regenerative medicine is anticipated to bestow considerable advantages to individuals affected by a broad array of pathologies. Placenta-derived mesenchymal stem cells exhibit flexibility and pleiotropic characteristics which incorporate immune system modulation and control of inflammation. Some of the properties like angiogenesis, neuroprotective and anti-apoptotic are the properties that have been broadly assessed at preclinical levels.[7],[38],[39] Owing to these properties, HPMCs possess huge potential as therapeutics against several diseases, which are illustrated in Figure 1 and have been discussed in detail in further sections.
Figure 1: Therapeutic applications of human placenta-derived mesenchymal stem cells against various diseases
SARS-CoV-2 is classified as a novel coronavirus that belongs to the β−coronavirus family. After Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), COVID-19 has emerged as the third zoonotic illness-based pandemic produced by viruses belonging to this family [40]. The genome of the COVID-19 virus is -26]-[32] kilobase pairs in length with crown-like projections on surfaces called spike proteins. The spike protein mainly interacts with the ACE-2 (angiotensin-converting enzyme) on alveolar epithelial cells and performs interspecies transmission of the genome.[41] This attachment leads to an elevated expression of ACE-2 which injures alveolar cells causing lesions. These lesions can cause serious systemic responses and eventually death if not treated on time. From affecting the lungs, these lesions can cause dysfunction of numerous organs.[42],[43] An immune destruction in the form of cytokine storm occurs in the body, in which pro-inflammatory cytokines, for example, interferons (INFs), interleukins (IL-1, 6, 12, 8, 33), tumor necrosis factors (TNFs), tumor growth factors (TGFs), accumulate in abundance.[44] MSC treatment can inhibit the immune system from releasing a flood of cytokines and boost endogenous healing about the stem cell’s reparative abilities. P-MSCs inhibit further infection of pulmonary alveolar cells by acting on the ACE-2 receptor and TMPRSS-2 (endothelial cell surface protein) receptor.[22] Placental MSC cells, when injected into the body, get entrapped in the lungs to restrict the systemic infusion of the virus. Thereafter, these MSCs have the potential to restore the required pulmonary environment, preserve alveolar epithelial cells, prevent pulmonary fibrosis, and treat lung dysfunction and COVID-19 pneumonia.[45] P-MSCs reduce the severity of cytokine storm caused by the virus, change the microenvironment to an anti-inflammatory state, and then further limit mononuclear cell entrance into the infected/damaged alveolar epithelium cells. In injured alveolar cells, P-MSCs increase immunomodulatory and anti-inflammatory properties. Thereby accelerating the repair mechanism of damaged alveolar cells.[22] Patients treated with MSC showed increased levels of peripheral lymphocytes and a decline in the amount of cytokine-secreting immune cells including CXCR3+ CD4+ T cells, CXCR3+ CD8+ T cells, and NK CXCR3+ cells compared with patients treated with conventional therapy.[45]
Cardiovascular disorders are a major cause of morbidity and death globally, with myocardial infarction (MI) being the most frequent cardiovascular disorder.[46] MI happens as a result of intervention in the circulation of blood to heart muscles which are accompanied by myocardial ischemia. This is influenced by the capability as well as the timing of the restoration of blood flow, and therefore, may or may not be reversible.[47] The impaired cardiac muscle is substituted by scar tissue as the restoration of the myocardium is practically not present, thereby limiting the functioning of the heart. Transplantation of placenta-derived mesenchymal stem cells acts as a promising approach for restoring heart function as well as lessening cardiac fibrosis because of the angiogenic and immunomodulatory characteristics possessed by them. These cells can potentially transform into myocardiocytes along with exhibiting voluntary movements in vitro environments indicating their therapeutic application in the process of heart repair.[7],[48],[49] Various researchers have inspected the influence of placenta-derived mesenchymal stem cells after transplanting them in animal models of MI. These stem cells when administered in the heart of rats after inducing MI exhibited incorporation into heart tissues and in vivo lineage programming to form myocardiocytes.[48] The prime route for arbitrating the relocation of mesenchymal stem cells in the vicinity of damaged tissues is the axis of chemokine receptor type 4 (CXCR4), and its ligand i.e., stromal cell-derived factor (SDF-1) forming CXCR4-SDF1. As observed, due to hypoxia, CXCR4 is instigated at a high extent in placental mesenchymal stem cells. Therefore, a huge chemotactic reaction is anticipated by the placental mesenchymal stem cells to the ischemic microenvironment of the damaged portion of the heart.[50] Injecting these stem cells intravenously in an infarcted rat manifested an upraised heart function after around 32 weeks from infarction.[51] Provisioning these stem cells with a novel ester of hyaluronan linked to butyric as well as retinoic acid (HBR) reinforces their restorative capability. Transplanting these provisioned stem cells in pigs generated a notable lowering in the extent of scar, more myocardial transmigration and glucose absorption, elevated density of capillaries, as well as a decline of fibrous tissues.[52] Researchers have also assessed the angiogenic paracrine effect of the conditioned medium of placental mesenchymal stem cells. In a rat model of reperfusion injury, injecting the conditioned medium of these stem cells reduced the size of infarction as well as myocardiocyte apoptosis, whilst encouraging the capillary density in the borderline of infarction.[53] Intracoronary injection of umbilical cord mesenchymal stem cells in people suffering from MI led to a healthy and markedly increased myocardial tolerance and circulation in the infarcted area. Enhancements in certain factors, like increased left ventricular ejection fraction as well as decreased end- diastolic volumes and LV end-systolic volumes, have been reported up until 18 months after therapy.[54] A phase I/II clinical study named RIMECARD showed the security and effectiveness of these stem cells which were injected intravenously in people suffering from chronic heart failure. There was also a decreased fraction ejection. Advancements in left ventricular activity, cognitive function, and life expectancy have been identified in treated groups.[55] The capacities of first trimester placental chorionic mesenchymal stem cells (FCMSCs) and third trimester placental chorionic mesenchymal stem cells (TCMSCs) have also been studied. FCMSCs had a better capacity to produce EC differentiation, as shown by improved in vitro morphology, angiogenic ability, in vivo cardiac function, and higher levels of pro- angiogenesis gene expression than TCMSCs.[56]
Critical limb ischemia is the most dangerous and advanced phase of peripheral artery disease which results from the thickening of walls of arteries due to the formation of plaque and brings scarcity in the blood perfusion, incorporating acute limb ischemia, gangrene, and even ulcers.[57],[58] It is accompanied by accelerating stenosis, and eventually leads to blockage of peripheral arteries. The risks of developing peripheral artery disease increase with advancing age, high lipid content such as cholesterol (hyperlipidemia), high blood pressure, and predominantly diabetes. The hostile tissue environment generated by diabetes and ischemia can impair the functioning of a patient’s cell products for autologous and allogeneic related therapies.[59] Angiogenic cells can contribute directly to the creation of new arteries while also providing endogenous growth factors, encouraging vascular expansion in a paracrine manner. Angiogenic treatment includes the use of angiogenic growth factors (HIF-1α, VEGF, FGF1, etc.), gene transfer techniques utilizing viral or non-viral vectors to transport a gene coding for a therapeutic protein to target tissues, or the use of angiogenic stem cells.[60] However, in several instances, amputation is the sole alternative possible for treating critical limb ischemia. This is because the blood capillaries cannot be rectified, and recompression of vessels is generated. It has been outlined in the preclinical trials that cell therapy exhibits several benefits in neovascularization in numerous mice suffering from hindlimb ischemia. Placental mesenchymal stem cells have illustrated pro-angiogenic properties, when introduced into the ischemic area of the infected limb via intramuscular injections, thereby enhancing the blood circulation as well as encouraging the development of new vessels.[24],[61],[62] PMSCs can be administered with insulin (from external sources) and even without insulin. Upon administration, the efficacy of PMSC was calculated. The results were promising as there were newly formed capillaries, a higher number of arterioles, and even high secretion of different proangiogenic factors.[63] Furthermore, conditioned medium from placental mesenchymal stem cells also possessed pro-angiogenic effects in a mouse model having hindlimb ischemia, equivalent to the transplanted placental stem cells in the same research, disclosing that action of placental mesenchymal stem cell led fundamentally from the paracrine effect of the angiogenic elements secreted from these stem cells.[24] Nonetheless, in one more examination, cells were more effective in comparison to the cell lysate in liberating blood circulation, presumably stipulating the significance of elongated paracrine action for optimal blood flow restoration.[63]
Ischemic stroke, also called a cerebrovascular accident, is acute damage in the central nervous system caused due to compromised vasculature or brain perfusion, incorporating cerebral infarction, cerebral bleed, and subarachnoid hemorrhage. It predominantly causes disability globally, and about 85% of strokes appear to be ischemic.[64] The frequently utilized curative technique for this condition is thrombolysis; however, many patients avoid out of window visits which is necessary for efficacious treatment. It has been outlined via experimental facts that regenerative medicine can lessen the degeneration of neurons as well as boost functional outcomes. Placental mesenchymal stem cells exhibit neuroprotective effects, which have been illustrated in stroke rat models. Administering these stem cells intravenously leads to a noteworthy enhancement of functional outcomes along with a considerable reduction in the mass of lesions, after 4 hours in the injury. This can be correlated with an escalation in the amounts of vascular endothelial and hepatocyte growth factors, as well as a brain-derived neurotrophic factor in the ischemic brain in comparison with the controls.[65] Similarly, stem cells can limit the injury caused by stroke. For the first time, Barzegar et al, (2021)[66] discovered that intravenous injection of placental MSCs administered at the moment of reperfusion dramatically reduced ischemia damage in the ipsilateral hemisphere. This protection is linked to a substantial return of normal blood flow to the brain following MCAO (middle cerebral artery occlusion).
Over the years, our understanding and ability to deal with cancer have improved significantly. Despite the accelerated progress in the oncology domain, cancer remains a major lethality affecting both developing and developed parts of the world. The conventional treatment of cancer including surgical removal of the tumor, radiotherapy, and chemotherapy, have proven to be effective, however, severe side effects and drug resistance highlight the need to find an alternate therapy that could minimize the toxicity and still be efficient in treating the tumor cells.[67] Placenta-derived mesenchymal stem cells offer a novel approach to cancer treatment. Placenta-derived MSCs have an intrinsic nature to migrate to the sites in the body which shows any kind of wear and tear. This property of PMSCs is majorly exploited in cancer treatment. Cancer and tumor sites behave as an unhealed wounded environment which sends a signal through paracrine signaling to PMSCs to migrate to the site of action. Inflammation is another factor that contributes to the movement of the cells.[68],[69] This migration property was first observed in a xenograft mouse model.[70] PMSCs can be used as cellular Trojan horses as well for the targeted drug delivery. Nanoparticles are a novel approach for drug delivery but being foreign particles, nanoparticles are prone to high immunogenic response and have a high rate of failure as well due to inefficient dissemination in tumors. All these can be overcome by using stem cells as their delivery system.[71],[72] This compound system of drug delivery has proven to be quite efficient in tumor apoptosis. MSC cell membrane coated with doxorubicin enclosed in porous silica nanoparticles is a fine example.[73] Furthermore, PMSCs can also be programmed genetically to produce certain antitumor compounds and peptides. Generally, PMSCs are modified with viral particles for the expression of certain cytokines like interferon-β (INF-β). A study conducted by Shen et al. showed induced apoptosis in breast cancer cells when umbilical cord MSCs were subjected to adenovirus particles expressing INF-β.[74] They can also be modified for producing particular enzymes as evident in the case of cytosine deaminase- producing MSCs when injected into the brain hindered the growth of glioblastoma.[70] Endostatin, a neurovascular and anti-angiogenesis inhibitor, has been successfully administered using hPMSCs, which works as a gene vector in the treatment of ovarian cancer.[75] Along with acting as an accessory to other forms of therapies, PMSCs as their own also have anti-tumorigenic responses. Studies have demonstrated MSCs secreting cytotoxic peptides, mediating pro-apoptotic pathways, and inducing an anti-cancer immune response. Growth of breast cancer was attenuated by the placental MSCs through inhibiting ERK1/2 and activating the AKT pathway, leading to apoptosis.[76] TNF-related apoptosis-inducing ligand (TRAIL) is another cytotoxic agent released by MSCs inducing apoptotic pathways, all leading to inhibiting the growth of several types of carcinomas.[77] Combining PMSCs with drugs to improve restrictions in treatment is also considered. Therapeutic medicines combining anti-angiogenic treatment and chemotherapy can be utilized with decidua-derived MSCs. The decidua mesenchymal stem cells were used to carry out a combination of endostatin transfection and doxorubicin-nanoparticle loading.[78]
In Parkinson’s disease (PD) a gradual impairment of motor skills can be observed. This happens when neurons in the brain controlling the locomotive functions become functionally inactive or die. It is characterized by the loss of dopamine-releasing neurons and thereby, decreased levels of dopamine in the striatum. There is no cure available for the full-fledged treatment of the disease, however, few medicines and therapies have been employed to target the symptoms. Anticholinergics, dopamine agonists, and neuroprotective (drug therapy) are the most common treatments for PD to date.[14],[79] When talking to stem cells, the main objective becomes to re-establish the dopaminergic neurons and related transmission signals. Neural progenitors produced by the differentiation of PMSCs were transplanted into a rat with PD. The results showed alleviation of the PD symptoms and the neural progenitors transformed into dopaminergic differentiation.[80] In a study on mice, MSCs can lower the amount of α-synuclein, block microglia cell polarization, and increase neuron survival.[81] It is believed and being studied that treatment with PMSCs can also help in symptoms unrelated to the motor like constipation and hyposmia through the secretion of anti-apoptotic and anti-inflammatory factors.
Alzheimer’s disease (AD) is yet another progressive disease that is characterized by dementia i.e., memory loss with aging. It is a result of abnormal accumulation of amyloid peptides and tau tangles all over the brain causing loss of neural activity.[79] Structurally, it usually starts with the hippocampus and entorhinal cortex, parts primarily responsible for memory response. With time, shrinkage in other parts of the brain is also visible. AD mice models injected with PMSCs demonstrated higher levels of amyloid breaking enzymes and also lower levels of pro-inflammatory peptides. PMSCs showed a strong immunomodulatory effect by increasing the levels of anti-inflammatory cytokines like IL-10 and TGF-β. Moreover, enhanced memory response was also observed.[82] MSCs generated from the placental amniotic membrane can help AD model mice enhance their spatial learning and memory. The number of amyloid plaques was also decreased, suggesting that MSC therapy generated from the human placental amniotic membrane might ameliorate AD pathogenesis and cognitive performance by regulating oxidative stress.[83]
Another neurological disease is amyotrophic lateral sclerosis (ALS), which is characterized by symptoms like muscle weakness, paralysis, respiratory problems ultimately leading to death. These symptoms are caused by the degeneration of neurons in the brain and spinal cord. Although no conclusive therapies are available for ALS, the stem cells put forward an approach that leads to extended lifespan. ALS mice models injected with PMSCs demonstrated delayed degeneration of motor neurons by shielding them from inflammatory peptides.[84] MSCs have been found to migrate to the spinal cord of mice and inhibit microglial activation and tissue glial proliferation, increasing the number of motor neurons, suggesting that MSCs may have neuroprotective properties. Experiments have demonstrated that exosomes produced from ASC can protect motor neuron-like NSC-34 cells from oxidative damage and improve cell viability.[14]
The pathogenicity of multiple sclerosis (MS) can be divided into two sections; inflammatory immune response and the degenerative process. The main characteristic of MS is the demyelinated portion in the central nervous system. The demyelination is healed by the formation of a glial scar. MS is also an auto- immune disorder mediated by the T-cells, attacking the myelin sheath peptides leading to loss of neurons and dendrocytes. Therapies available and in the study mostly target the immune system. PMSCs provide a unique approach to the treatment of MS patients by regulating both the immune response and facilitating the regeneration of the neural cells. Many studies have been conducted which use PMSCs on autoimmune encephalomyelitis (EAE) mouse models. The symptoms of EAE closely resemble MS symptoms. An immune response is triggered by the injection of myelin antigen and transplantation of PMSCs at 5th day and 14th day from immunization was concluded to reduce disease lethality and extended survival.[85] Injection of PMSCs is the EAE mice models were also an effective approach as these delayed the motor symptoms, modulated immune response by inhibiting the T cell proliferation and reducing inflammatory reactions, preventing neural network loss, and decreasing the seriousness of MS.[86],[87] The therapeutic efficacy of transplanting these stem cells in patients suffering from MS has been tested in various trials. Injecting umbilical cord-derived mesenchymal stem cells intravenously comes out to be feasible and safe in such patients. Additionally, the overall effects on treatment groups were observed to be stable or increased in comparison to the standard group.[88] In another research, patients having relapsing-remitting MS or secondary progressive MS were spontaneously administered with placental stem cells (PDA-001, a preparation of hPMSCs) and several individuals who were treated had steady or declining expanded disability status scale ratings (EDSS), which showed potential reparative influence on the patients.[89]
Cirrhosis is the final stage of many liver injuries like viral infections, metabolic disorders, alcoholism, and even acute liver failure resulting from various processes. The typical functioning of the liver is disturbed due to the formation of the scar by the extracellular matrix. It further leads to necroinflammation and fibrogenesis.[90],[91] Liver cirrhosis is an irrevocable condition that can be deadly, and the sole option for curing the disease is liver transplantation. However, the lesser availability of donors along with the constant requirement of immunosuppressants restrict liver transplantation and this is why cell transplantation emerges to be an appropriate alternative. Besides fetal and adult hepatocytes, stem cells are also taken into consideration for cell transplantations. It has been outlined that PMSCs can potentially be beneficent because of their ability to transform to hepatic-like cells, thereby forming functioning 3D structures.[92] AF-MSCs can engraft in the injured liver and produce Annexin-A1 and other helpful cytokines to successfully reduce the degree of liver damage and promote liver repair and regeneration.[93] Vascular cell adhesion molecule-1 and very late antigen-4 adhesion molecules help PMSCs to concentrate in injured areas. Through cell-cell interactions and the production of growth factors including hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), PMSCs help to heal the injured liver.[94],[95] These stem cells have been shown to induce a notable decrease in fibrosis and also in the serum amounts of transaminases. An enhancement in liver regeneration has been suggested due to initiation in the mechanism of autophagy[96], a decline in apoptosis, repression in the activation of stellate cells[97], and incitement in liver cell proliferation.[98] They are also shown to limit hepatocyte inflammation as well as prevent apoptosis by suppression of TNF-α and IFN-γ. This causes the rejuvenation of hepatocytes and release of HGF, VEGF, IL-6, and PAF (Platelet-activating factor)[75],[99]. No indications regarding the formation of hepatocytes from cell transplants have been revealed in the rat model exhibiting CC14-induced fibrosis98, however, engraftment of PMSCs along with human albumin and α-fetoprotein expression has been outlined.[97],[100],[101]
Although bones have a physiological capability of regeneration and remodeling as evident from the healing of bones in case of any damage, there are some physiological scenarios when the physical regeneration of bone is compromised and insufficient. Osteoporosis is one of the conditions where compromised regeneration is seen. Deployment of PMSCs as a therapy for bone disorders exploits their multipotency. PMSCs possess the potential to differentiate and transform into osteocytes. They are in vivo potential of forming bones have been acknowledged by many studies thereby making the therapy an interesting candidate to facilitate bone repair. In a study, PMSCs injected in mice models with femur defects were able to fully recover the damage without any signs of fibrosis. They were injected in combination with nano biphasic calcium phosphate ceramics.[102] Another study used hydroxyapatite/tricalcium phosphate particles to form bones at the implants in a severe immune deficiency (SCID) mice model.[103] The multipotency and ability to differentiate into musculoskeletal lineages makes PMSC an alternative and interesting option for osteoarthritis (OA), a degenerative disease of cartilages in the joints. Many studies have been conducted supporting the regenerative hypothesis using PMSCs. Rat models with osteochondral defects in the knee were found to form hyaline cartilage when administered with the PMSCs grown on silk fibroin.[104] In a study, for covering the femoral defects, PMSCs infused in collagen I gel were subjected to a diseased rat model. Upon testing, the soft-grown tissue was positive for toluidine blue which indicated the differentiation of transplanted cells.[105] Proliferative and osteogenic effects of andrographolide (AP) on human-PMSCs have also been studied. It also improved osteogenic differentiation by increasing the expression of osteoblast-specific mRNA. Furthermore, it can aid bone development by increasing the amount of the bone structural protein osteocalcin in osteoblastic cells.[106]
Crohn’s disease, as well as ulcerative colitis, are chronic illnesses triggered due to prolonged swelling of the epithelial cells in the intestinal tract leading to tissue damage all over the gastrointestinal system. These conditions are thought to be a consequence of an aberrant immunological response to intra- luminal antigens among susceptible individuals. Many other specific gene mutations of the nucleotide- binding oligomerization domain [2] (NOD2) are involved in the progression of this disorder.[107] These two illnesses have significant effects on well-being and presently no cure is available. In addition, most sufferers are not receptive to early detection and treatment. Injecting a conditioned medium of PMSCs intraperitoneally improved clinical characteristics in a mouse paradigm suffering from colitis instigated by dextran sulfate sodium.[108] These stem cells when injected intraperitoneally also inhibited tissue necrosis and reduced mouse fatalities. These positive effects seemed to be significantly higher while using NOD2-activated stem cells.[82] A study showed the safety of intravenous infusion of these stem cells (PDA001) in individuals suffering from intermediate to serious level Crohn’s disorder, and subsequently, certain recovery rates of the illness were also noted.[109] Similarly, in another monitored clinical experiment, the patient’s situation enhanced dramatically on intravenously injecting these stem cells. This led to a considerable decrease in steroid concentrations. Furthermore, many patients having anal fistula exhibited significant progress.[110]
Clinical trials are human research studies that are used to assess the effectiveness of pharmacological, surgical, or cognitive interventions. PMSCs have also undergone clinical trials for the treatment of certain diseases. For instance, a Phase 1 study has been conducted for determining the safety of intramuscular dosage of PLX-PAD (placenta-derived MSC) for treating critical limb ischemia. Similarly, recently another phase 1 and 2 clinical trials are being conducted for assessing the potential of cryopreserved PMSCs against deadly SARS-COV-2. Many such registered clinical levels are mentioned in Table 1.
Table 1: List of registered clinical trials of placenta-derived mesenchymal stem cells for treating various diseases.[111]
| NCT Number | Purpose of Study | Disease | Phase |
|---|---|---|---|
| NCT00919958 (YOS-2009) |
Determining the safety of PLX-PAD (placenta- derived MSC) single dose, intramuscular injection for the treatment of CLI |
Critical limb ischemia | Phase 1 |
| NCT04464213 (YOS-2020) |
Evaluate the efficacy and safety of human PMSCs on diabetic foot ulcer |
Diabetic foot ulcer | Phase 1 |
| NCT04464213 (YOS-2020) |
Study the pharmacokinetics and tolerance of hPMSC on diabetic foot ulcer |
Diabetic foot ulcer | Phase 1 |
| NCT01420432 (YOS-2011) |
Safety and efficacy study of umbilical cord/PMSCs to treat ankylosing spondylitis |
Ankylosing spondylitis (inflammatory arthritis) |
Phase 1 |
| NCT01413035 (2011) |
Evaluate the safety and efficacy of hPMSCs at a dose of 1.0E+6 MSC/kg for type 2 diabetes |
Type 2 diabetes | Phase 1 and 2 |
| NCT01413035 ( 2011) |
Evaluate the safety and efficacy of MSCs derived from the human placenta at a dose of 1.0E+6 MSC/kg for type 2 diabetes |
Type 2 diabetes | Phase 1 and 2 |
| NCT04652908 (2020) |
Study the efficacy of treatment of human placenta-derived MSCs seeded on a commercially available dural graft extracellular matrix against myelomeningocele |
Myelomeningocele | Phase 1 and 2 (ongoing) |
| NCT01129739 (2010) |
Evaluate safety and efficacy of hPMSCs at a dose of 1.0E+6 MSC/kg on subjects for refractory anemia (RA) and refractory anemia with ring sideroblast (RARS) of myelodysplastic syndromes (MDS) |
Myelodysplastic syndromes (a type of cancer) |
Phase 2 |
Placenta derived mesenchymal stem cells serve as potential sources for use in stem cell therapy in humans. Cell therapy employing these stem cells is primarily based on three essential properties they possess, i.e., their innate remedial ability or the release of paracrine factors, their ability to homing and engraftment and immune regulation capacity. Conversely, the medical utilization of PMSCs is still in its infant stages and many trials are still under research. Many trails involving cell therapy have been conducted using autologous stem cells. However, there are many drawbacks to using a patient’s own cells. Firstly, there is a time constraint, as the growth and quality control of autologous cells can take some months. In addition, cells may exhibit lesser efficacy owing to inherent elderly factors, and these PMSCs pose some attributes which can make autologous gene therapy virtually impossible, as observed in the case of older patients as well as those with particular systemic chronic conditions such as diabetes. Allogeneic mesenchymal stem cells, on the other hand, have the ability to be produced in high yields quickly so that they can be easily accessible and delivered instantly. They can be procured under more systematic and explicitly tested parameters and are likely to minimize costs. Till now, published reports on the efficacy of therapy using PMSCs suggest that these cells are safe and thus they are already ‘off-the- shelf’ products. While many clinical studies are underway or provide no published findings, there are a few positive reports on effectiveness of this treatment. Regenerative nanomedicine is indeed a propitious field which has provided very satisfactory outcomes at pre-clinical levels. Therapies of many illnesses can profit with the utilization of scaffolds, which provide a three-dimensional framework to facilitate cells, promote its adherence and development, thereby improve engraftment and subsequently the therapeutic outcomes. Apart from the use of cells as transporters of nanomaterials to transport medications within the wounded tissue and, even more, the prospect of stimulus-controlled secretion of medicines appears to be exhilarating. Along the way, further research is required on these stem cells which can potentially give promising results in the field of regenerative medicine.
The authors are grateful to the Delhi Technological University, New Delhi, India for providing the opportunity and support to conduct this study.
The author declares that there are no conflicts of interest relevant to this article.
References
- 1.Hipp J, Atala A. Sources of Stem Cells for Regenerative Medicine. Stem Cell Rev. 2008 Mar 20;4(1):3–11. [Google Scholar] [PubMed]
- 2.Quartu M, Serra MP, Boi M, Ibba V, Melis T, Del Fiacco M. Polysialylated-neural cell adhesion molecule (PSA-NCAM) in the human trigeminal ganglion and brainstem at prenatal and adult ages. BMC Neurosci. 2008 Dec 6;9(1):108. [Google Scholar] [PubMed]
- 3.Yen BL, Huang H-I, Chien C-C, Jui H-Y, Ko B-S, Yao M, et al. Isolation of Multipotent Cells from Human Term Placenta. Stem Cells. 2005 Jan;23(1):3–9. [Google Scholar] [PubMed]
- 4.Lee OK, Kuo TK, Chen W-M, Lee K-D, Hsieh S-L, Chen T-H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004 Mar 1;103(5):1669–75. [Google Scholar] [PubMed]
- 5.Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front Physiol. 2018 Aug 17;9. [Google Scholar] [PubMed]
- 6.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019 Dec 26;10(1):68. [Google Scholar] [PubMed]
- 7.Macias MI, Grande J, Moreno A, Domínguez I, Bornstein R, Flores AI. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am J Obstet Gynecol. 2010 Nov;203(5):495.e9-495.e23. [Google Scholar] [PubMed]
- 8.Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GMJS, Claas FHJ, Fibbe WE, et al. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells. 2004 Dec;22(7):1338–45. [Google Scholar] [PubMed]
- 9.Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human Placenta-Derived Cells Have Mesenchymal Stem/Progenitor Cell Potential. Stem Cells. 2004 Sep;22(5):649–58. [Google Scholar] [PubMed]
- 10.Yen BL, Chien C-C, Chen Y-C, Chen J-T, Huang J-S, Lee F-K, et al. Placenta-Derived Multipotent Cells Differentiate into Neuronal and Glial Cells In Vitro. Tissue Eng Part A. 2008 Jan;14(1):9–17. [Google Scholar] [PubMed]
- 11.Chang C-M, Kao C-L, Chang Y-L, Yang M-J, Chen Y-C, Sung B-L, et al. Placenta-derived multipotent stem cells induced to differentiate into insulin-positive cells. Biochem Biophys Res Commun. 2007 Jun;357(2):414–20. [Google Scholar] [PubMed]
- 12.Chien C-C, Yen BL, Lee F-K, Lai T-H, Chen Y-C, Chan S-H, et al. In Vitro Differentiation of Human Placenta- Derived Multipotent Cells into Hepatocyte-Like Cells. Stem Cells. 2006 Jul;24(7):1759–68. [Google Scholar] [PubMed]
- 13.Lee JM, Jung J, Lee H-J, Jeong SJ, Cho KJ, Hwang S-G, et al. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012 Jun;13(2):219–24. [Google Scholar] [PubMed]
- 14.Yao P, Zhou L, Zhu L, Zhou B, Yu Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur Neurol. 2020;83(3):235–41. [Google Scholar] [PubMed]
- 15.Ns S, Na A. Sources of Mesenchymal Stromal Cells : An Overview. Am Jorunal Pharmacol. 2018;1(1):1–7. [Google Scholar] [PubMed]
- 16.Yun Cheng H. The Impact of Mesenchymal Stem Cell Source on Proliferation, Differentiation, Immunomodulation and Therapeutic Efficacy. J Stem Cell Res Ther. 2014;04(10). [Google Scholar] [PubMed]
- 17.Pipino C, Shangaris P, Resca E, Zia S, Deprest J, Sebire NJ, et al. Placenta as a reservoir of stem cells: an underutilized resource? Br Med Bull. 2013 Mar 1;105(1):43–68. [Google Scholar] [PubMed]
- 18.Kim EY, Lee K-B, Kim MK. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014 Mar 31;47(3):135–40. [Google Scholar] [PubMed]
- 19.Siddesh SE, Gowda DM, Jain R, Gulati A, Patil GS, Anudeep TC, et al. Placenta-derived mesenchymal stem cells (P-MSCs) for COVID-19 pneumonia-a regenerative dogma. Stem Cell Investig. 2021 Feb;8:3–3. [Google Scholar] [PubMed]
- 20.Woods L, Perez-Garcia V, Hemberger M. Regulation of Placental Development and Its Impact on Fetal Growth—New Insights From Mouse Models. Front Endocrinol (Lausanne). 2018 Sep 27;9. [Google Scholar] [PubMed]
- 21.Abbasi-Kangevari M, Ghamari S-H, Safaeinejad F, Bahrami S, Niknejad H. Potential Therapeutic Features of Human Amniotic Mesenchymal Stem Cells in Multiple Sclerosis: Immunomodulation, Inflammation Suppression, Angiogenesis Promotion, Oxidative Stress Inhibition, Neurogenesis Induction, MMPs Regulation, and Remyelination Stim. Front Immunol. 2019 Feb 20;10. [Google Scholar] [PubMed]
- 22.Siddesh SE, Gowda DM, Jain R, Gulati A, Patil GS, Anudeep TC, et al. Placenta-derived mesenchymal stem cells (P-MSCs) for COVID-19 pneumonia-a regenerative dogma. Stem Cell Investig. 2021 Jul 9;8. [Google Scholar] [PubMed]
- 23.Toda A, Okabe M, Yoshida T, Nikaido T. The Potential of Amniotic Membrane/Amnion-Derived Cells for Regeneration of Various Tissues. J Pharmacol Sci. 2007;105(3):215–28. [Google Scholar] [PubMed]
- 24.Ambrósio CE, Orlandin JR, Oliveira VC, Motta LCB, Pinto PAF, Pereira VM, et al. Potential application of aminiotic stem cells in veterinary medicine. Anim Reprod. 2020 May 22;16(1):24–30. [Google Scholar] [PubMed]
- 25.Portmann-Lanz CB, Schoeberlein A, Huber A, Sager R, Malek A, Holzgreve W, et al. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am J Obstet Gynecol. 2006 Mar;194(3):664–73. [Google Scholar] [PubMed]
- 26.Ventura Ferreira MS, Bienert M, Müller K, Rath B, Goecke T, Opländer C, et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res Ther. 2018 Dec 5;9(1):28. [Google Scholar] [PubMed]
- 27.L. PK, Kandoi S, Misra R, S. V, K. R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019 Apr;46:1–9. [Google Scholar] [PubMed]
- 28.Bailo M, Soncini M, Vertua E, Signoroni PB, Sanzone S, Lombardi G, et al. Engraftment Potential of Human Amnion and Chorion Cells Derived from Term Placenta. Transplantation. 2004 Nov 27;78(10):1439–48. [Google Scholar] [PubMed]
- 29.Vegh I, Grau M, Gracia M, Grande J, de la Torre P, Flores AI. Decidua mesenchymal stem cells migrated toward mammary tumors in vitro and in vivo affecting tumor growth and tumor development. Cancer Gene Ther. 2013 Jan 5;20(1):8–16. [Google Scholar] [PubMed]
- 30.Pogozhykh O, Prokopyuk V, Figueiredo C, Pogozhykh D. Placenta and Placental Derivatives in Regenerative Therapies: Experimental Studies, History, and Prospects. Stem Cells Int. 2018;2018:1–14. [Google Scholar] [PubMed]
- 31.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ Res. 2008 Nov 21;103(11):1204–19. [Google Scholar] [PubMed]
- 32.Taglauer ES, Adams Waldorf KM, Petroff MG. The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance. Int J Dev Biol. 2010;54(2–3):421–30. [Google Scholar] [PubMed]
- 33.Blaschitz A, Hutter H, Dohr G. HLA ClassI protein expression in the human placenta. Early Pregnancy. 2001 Jan;5(1):67–9. [Google Scholar] [PubMed]
- 34.Xu X, Zhou Y, Wei H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front Immunol. 2020 Oct 22;11. [Google Scholar] [PubMed]
- 35.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002 Apr;23(4):239–56. [Google Scholar] [PubMed]
- 36.McCracken SA, Hadfield K, Rahimi Z, Gallery ED, Morris JM. NF-κB-regulated suppression of T-bet in T cells represses Th1 immune responses in pregnancy. Eur J Immunol. 2007 May;37(5):1386–96. [Google Scholar] [PubMed]
- 37.Heikkinen J, Möttönen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol. 2004 May;136(2):373–8. [Google Scholar] [PubMed]
- 38.Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, et al. Human Placental Mesenchymal Stem Cells (pMSCs) Play a Role as Immune Suppressive Cells by Shifting Macrophage Differentiation from Inflammatory M1 to Anti-inflammatory M2 Macrophages. Stem Cell Rev Reports. 2013 Oct 28;9(5):620–41. [Google Scholar] [PubMed]
- 39.Yust-Katz S, Fisher-Shoval Y, Barhum Y, Ben-Zur T, Barzilay R, Lev N, et al. Placental mesenchymal stromal cells induced into neurotrophic factor-producing cells protect neuronal cells from hypoxia and oxidative stress. Cytotherapy. 2012 Jan;14(1):45–55. [Google Scholar] [PubMed]
- 40.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N Engl J Med. 2020 Mar 26 [cited 2021 Jul 4];382(13):1199–207. [Google Scholar] [PubMed]
- 41.Madabhavi I, Sarkar M, Kadakol N. COVID-19. A review. Monaldi Arch Chest Dis. 2020 May 14;90(2). [Google Scholar] [PubMed]
- 42.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020 Jun 5;92(6):548–51. [Google Scholar] [PubMed]
- 43.Luthra R, Kaur S, Bhandari K. Applications of CRISPR as a potential therapeutic. Life Sci. 2021 Nov;284:119908. [Google Scholar] [PubMed]
- 44.Iwata-Yoshikawa N, Okamura T, Shimizu Y, Hasegawa H, Takeda M, Nagata N. TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. Gallagher T, editor. J Virol. 2019 Mar 15;93(6). [Google Scholar] [PubMed]
- 45.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020 Mar 9;11(2):216-228. [Google Scholar] [PubMed]
- 46.Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int J Cardiol. 2013 Sep;168(2):934–45. [Google Scholar] [PubMed]
- 47.Faiella W, Atoui R. Therapeutic use of stem cells for cardiovascular disease. Clin Transl Med. 2016 Dec 18;5(1). [Google Scholar] [PubMed]
- 48.Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human Amniotic Mesenchymal Cells Have Some Characteristics of Cardiomyocytes. Transplantation. 2005 Mar 15;79(5):528–35. [Google Scholar] [PubMed]
- 49.Okamoto K, Miyoshi S, Toyoda M, Hida N, Ikegami Y, Makino H, et al. ‘Working’ cardiomyocytes exhibiting plateau action potentials from human placenta-derived extraembryonic mesodermal cells. Exp Cell Res. 2007 Jul;313(12):2550–62. [Google Scholar] [PubMed]
- 50.Li L, Jaiswal PK, Makhoul G, Jurakhan R, Selvasandran K, Ridwan K, et al. Hypoxia modulates cell migration and proliferation in placenta-derived mesenchymal stem cells. J Thorac Cardiovasc Surg. 2017 Aug;154(2):543-552.e3. [Google Scholar] [PubMed]
- 51.Lpez Y, Lutjemeier B, Seshareddy K, M. Trevino E, Sue Hageman K, I. Musch T, et al. Wharton’s Jelly or Bone Marrow Mesenchymal Stromal Cells Improve Cardiac Function Following Myocardial Infarction for More Than 32 Weeks in a Rat Model: A Preliminary Report. Curr Stem Cell Res Ther. 2013 Feb 1;8(1):46– 59. [Google Scholar] [PubMed]
- 52.Simioniuc A, Campan M, Lionetti V, Marinelli M, Aquaro GD, Cavallini C, et al. Placental stem cells pre- treated with a hyaluronan mixed ester of butyric and retinoic acid to cure infarcted pig hearts: a multimodal study. Cardiovasc Res. 2011 Jun 1;90(3):546–56. [Google Scholar] [PubMed]
- 53.Danieli P, Malpasso G, Ciuffreda MC, Cervio E, Calvillo L, Copes F, et al. Conditioned Medium From Human Amniotic Mesenchymal Stromal Cells Limits Infarct Size and Enhances Angiogenesis. Stem Cells Transl Med. 2015 May;4(5):448–58. [Google Scholar] [PubMed]
- 54.Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, et al. Intracoronary infusion of Wharton’s jelly- derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015 Dec 10;13(1):162. [Google Scholar] [PubMed]
- 55.Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure. Circ Res. 2017 Oct 27;121(10):1192–204. [Google Scholar] [PubMed]
- 56.Yu S, You X, Liang H, Li Y, Fu Y, Zhang X, Hu X, An J, Xu Y, Li F. First trimester placental mesenchymal stem cells improve cardiac function of rat after myocardial infarction via enhanced neovascularization. Heliyon. 2021 Feb 1;7(1):e06120. [Google Scholar] [PubMed]
- 57.Sprengers RW, Teraa M, Moll FL, de Wit GA, van der Graaf Y, Verhaar MC. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J Vasc Surg. 2010 Oct;52(4):843-849.e1. [Google Scholar] [PubMed]
- 58.Xie B, Luo H, Zhang Y, Wang Q, Zhou C, Xu D. Autologous Stem Cell Therapy in Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2018;2018:1–12. [Google Scholar] [PubMed]
- 59.Gu Y, Rampin A, Alvino V V., Spinetti G, Madeddu P. Cell Therapy for Critical Limb Ischemia: Advantages, Limitations, and New Perspectives for Treatment of Patients with Critical Diabetic Vasculopathy. Curr Diab Rep. 2021 Mar 2;21(3):11. [Google Scholar] [PubMed]
- 60.Beltrán-Camacho L, Rojas-Torres M, Durán-Ruiz MC. Current Status of Angiogenic Cell Therapy and Related Strategies Applied in Critical Limb Ischemia. Int J Mol Sci. 2021 Feb 26;22(5):2335. [Google Scholar] [PubMed]
- 61.Prather WR, Toren A, Meiron M, Ofir R, Tschope C, Horwitz EM. The role of placental-derived adherent stromal cell (PLX-PAD) in the treatment of critical limb ischemia. Cytotherapy. 2009 Jan;11(4):427–34. [Google Scholar] [PubMed]
- 62.Xie N, Li Z, Adesanya TM, Guo W, Liu Y, Fu M, et al. Transplantation of placenta-derived mesenchymal stem cells enhances angiogenesis after ischemic limb injury in mice. J Cell Mol Med. 2016 Jan 18;20(1):29–37. [Google Scholar] [PubMed]
- 63.Liang L, Li Z, Ma T, Han Z, Du W, Geng J, et al. Transplantation of Human Placenta-Derived Mesenchymal Stem Cells Alleviates Critical Limb Ischemia in Diabetic Nude Rats. Cell Transplant. 2017 Jan 1;26(1):45– 61. [Google Scholar] [PubMed]
- 64.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics-2016 Update. Circulation. 2016 Jan 26;133(4). [Google Scholar] [PubMed]
- 65.Zahavi-Goldstein E, Blumenfeld M, Fuchs-Telem D, Pinzur L, Rubin S, Aberman Z, et al. Placenta-derived PLX-PAD mesenchymal-like stromal cells are efficacious in rescuing blood flow in hind limb ischemia mouse model by a dose- and site-dependent mechanism of action. Cytotherapy. 2017 Dec;19(12):1438– 46. [Google Scholar] [PubMed]
- 66.Barzegar M, Wang Y, Eshaq RS, Yun JW, Boyer CJ, Cananzi SG, et al. Human placental mesenchymal stem cells improve stroke outcomes via extracellular vesicles-mediated preservation of cerebral blood flow. EBioMedicine. 2021 Jan;63:103161. [Google Scholar] [PubMed]
- 67.Luthra R, Datta S, Roy A. Role of Different Peptides for Cancer Immunotherapy. Int J Pept Res Ther. 2021 Sep 23. [Google Scholar] [PubMed]
- 68.Moodley Y, Vaghjiani V, Chan J, Baltic S, Ryan M, Tchongue J, et al. Anti-Inflammatory Effects of Adult Stem Cells in Sustained Lung Injury: A Comparative Study. Mezey E, editor. PLoS One. 2013 Aug 1;8(8):e69299. [Google Scholar] [PubMed]
- 69.Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008 May 10;15(10):730–8. [Google Scholar] [PubMed]
- 70.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc Natl Acad Sci. 2000 Nov 7;97(23):12846–51. [Google Scholar] [PubMed]
- 71.Auffinger B, Morshed R, Tobias A, Cheng Y, Ahmed AU, Lesniak MS. Drug-Loaded Nanoparticle Systems And Adult Stem Cells: A Potential Marriage For The Treatment Of Malignant Glioma? Oncotarget. 2013 Mar 31;4(3):378–96. [Google Scholar] [PubMed]
- 72.Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU, et al. Neural Stem Cell-Mediated Intratumoral Delivery of Gold Nanorods Improves Photothermal Therapy. ACS Nano. 2014 Dec 23;8(12):12450–60. [Google Scholar] [PubMed]
- 73.Li L, Guan Y, Liu H, Hao N, Liu T, Meng X, et al. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS Nano. 2011 Sep 27;5(9):7462–70. [Google Scholar] [PubMed]
- 74.Shen C-J, Chan T-F, Chen C-C, Hsu Y-C, Long C-Y, Lai C-S. Human umbilical cord matrix-derived stem cells expressing interferon-β gene inhibit breast cancer cells via apoptosis. Oncotarget. 2016 Jun 7;7(23):34172-9. [Google Scholar] [PubMed]
- 75.Zhang G-Z, Sun H-C, Zheng L-B, Guo J-B, Zhang X-L. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol. 2017 Dec 14;23(46):8152–68. [Google Scholar] [PubMed]
- 76.Ganta C, Chiyo D, Ayuzawa R, Rachakatla R, Pyle M, Andrews G, et al. Rat Umbilical Cord Stem Cells Completely Abolish Rat Mammary Carcinomas with No Evidence of Metastasis or Recurrence 100 Days Post–Tumor Cell Inoculation. Cancer Res. 2009 Mar 1;69(5):1815–20. [Google Scholar] [PubMed]
- 77.Stuckey DW, Shah K. TRAIL on trial: preclinical advances in cancer therapy. Trends Mol Med. 2013 Nov;19(11):685–94. [Google Scholar] [PubMed]
- 78.De la Torre P, Paris JL, Fernández-de la Torre M, Vallet-Regí M, Flores AI. Endostatin Genetically Engineered Placental Mesenchymal Stromal Cells Carrying Doxorubicin-Loaded Mesoporous Silica Nanoparticles for Combined Chemo- and Antiangiogenic Therapy. Pharmaceutics. 2021 Feb 10;13(2):244. [Google Scholar] [PubMed]
- 79.Luthra R, Roy A. Role of medicinal plants against neurodegenerative diseases. Curr Pharm Biotechnol. 2021 Feb 12;22. [Google Scholar] [PubMed]
- 80.Park S, Kim E, Koh S-E, Maeng S, Lee W, Lim J, et al. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012 Jul;1466:158–66. [Google Scholar] [PubMed]
- 81.Shin JY, Lee PH. Mesenchymal stem cells modulate misfolded α-synuclein in parkinsonian disorders: A multitarget disease-modifying strategy. Stem Cell Res. 2020 Aug;47:101908. [Google Scholar] [PubMed]
- 82.Kim H, Shin T, Lee B, Yu K, Seo Y, Lee S, et al. Human Umbilical Cord Blood Mesenchymal Stem Cells Reduce Colitis in Mice by Activating NOD2 Signaling to COX2. Gastroenterology. 2013 Dec;145(6):1392- 1403.e8. [Google Scholar] [PubMed]
- 83.Jiao H, Shi K, Zhang W, Yang L, Yang L, Guan F, et al. Therapeutic potential of human amniotic membrane- derived mesenchymal stem cells in APP transgenic mice. Oncol Lett. 2016 Sep;12(3):1877–83. [Google Scholar] [PubMed]
- 84.Garbuzova-Davis S, Rodrigues MCO, Mirtyl S, Turner S, Mitha S, Sodhi J, et al. Multiple Intravenous Administrations of Human Umbilical Cord Blood Cells Benefit in a Mouse Model of ALS. Mosley RL, editor. PLoS One. 2012 Feb 3;7(2):e31254. [Google Scholar] [PubMed]
- 85.Fisher-Shoval Y, Barhum Y, Sadan O, Yust-Katz S, Ben-Zur T, Lev N, et al. Transplantation of Placenta- Derived Mesenchymal Stem Cells in the EAE Mouse Model of MS. J Mol Neurosci. 2012 Sep 26;48(1):176– 84. [Google Scholar] [PubMed]
- 86.Bravo B, Gallego MI, Flores AI, Bornstein R, Puente-Bedia A, Hernández J, et al. Restrained Th17 response and myeloid cell infiltration into the central nervous system by human decidua-derived mesenchymal stem cells during experimental autoimmune encephalomyelitis. Stem Cell Res Ther. 2016 Dec 17;7(1):43. [Google Scholar] [PubMed]
- 87.Jiang H, Zhang Y, Tian K, Wang B, Han S. Amelioration of experimental autoimmune encephalomyelitis through transplantation of placental derived mesenchymal stem cells. Sci Rep. 2017 Mar 10;7(1):41837. [Google Scholar] [PubMed]
- 88.Li J-F, Zhang D-J, Geng T, Chen L, Huang H, Yin H-L, et al. The Potential of Human Umbilical Cord-Derived Mesenchymal Stem Cells as a Novel Cellular Therapy for Multiple Sclerosis. Cell Transplant. 2014 Jan 1;23(1_suppl):113–22. [Google Scholar] [PubMed]
- 89.Lublin FD, Bowen JD, Huddlestone J, Kremenchutzky M, Carpenter A, Corboy JR, et al. Human placenta- derived cells (PDA-001) for the treatment of adults with multiple sclerosis: A randomized, placebo- controlled, multiple-dose study. Mult Scler Relat Disord. 2014 Nov;3(6):696–704. [Google Scholar] [PubMed]
- 90.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008 Mar;371(9615):838–51. [Google Scholar] [PubMed]
- 91.Williams R. Sherlock’s disease of the liver and biliary systems. Clin Med (Northfield Il). 2011 Oct 1;11(5):506–506. [Google Scholar] [PubMed]
- 92.Bornstein R, Macias MI, de la Torre P, Grande J, Flores AI. Human decidua-derived mesenchymal stromal cells differentiate into hepatic-like cells and form functional three-dimensional structures. Cytotherapy. 2012 Sep;14(10):1182–92. [Google Scholar] [PubMed]
- 93.Zagoura D, Trohatou O, Makridakis M, Kollia A, Kokla N, Mokou M, et al. Functional secretome analysis reveals Annexin-A1 as important paracrine factor derived from fetal mesenchymal stem cells in hepatic regeneration. EBioMedicine. 2019 Jul;45:542–52. [Google Scholar] [PubMed]
- 94.Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015 Aug 27;30(5):580–9. [Google Scholar] [PubMed]
- 95.Luan Y, Kong X, Feng Y. Mesenchymal stem cells therapy for acute liver failure: Recent advances and future perspectives. Liver Res. 2021 Jun;5(2):53–61. [Google Scholar] [PubMed]
- 96.Jung J, Choi JH, Lee Y, Park J-W, Oh I-H, Hwang S-G, et al. Human Placenta-Derived Mesenchymal Stem Cells Promote Hepatic Regeneration in CCl 4 -Injured Rat Liver Model via Increased Autophagic Mechanism. Stem Cells. 2013 Aug;31(8):1584–96. [Google Scholar] [PubMed]
- 97.Zhang D, Jiang M, Miao D. Transplanted Human Amniotic Membrane-Derived Mesenchymal Stem Cells Ameliorate Carbon Tetrachloride-Induced Liver Cirrhosis in Mouse. Aziz S, editor. PLoS One. 2011 Feb 4;6(2):e16789. [Google Scholar] [PubMed]
- 98.Tsai P-C, Fu T-W, Chen Y-MA, Ko T-L, Chen T-H, Shih Y-H, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transplant. 2009 May;15(5):484–95. [Google Scholar] [PubMed]
- 99.Fiore EJ, Bayo JM, Garcia MG, Malvicini M, Lloyd R, Piccioni F, et al. Mesenchymal Stromal Cells Engineered to Produce IGF-I by Recombinant Adenovirus Ameliorate Liver Fibrosis in Mice. Stem Cells Dev. 2015 Mar 15;24(6):791–801. [Google Scholar] [PubMed]
- 100.Cao H, Yang J, Yu J, Pan Q, Li J, Zhou P, et al. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012 Dec 6;10(1):56. [Google Scholar] [PubMed]
- 101.Yu J, Hao G, Wang D, Liu J, Dong X, Sun Y, et al. Therapeutic Effect and Location of GFP-Labeled Placental Mesenchymal Stem Cells on Hepatic Fibrosis in Rats. Stem Cells Int. 2017;2017:1–11. [Google Scholar] [PubMed]
- 102.Reddy S, Wasnik S, Guha A, Kumar JM, Sinha A, Singh S. Evaluation of nano-biphasic calcium phosphate ceramics for bone tissue engineering applications: In vitro and preliminary in vivo studies. J Biomater Appl. 2013 Jan 27;27(5):565–75. [Google Scholar] [PubMed]
- 103.Kusuma GD, Menicanin D, Gronthos S, Manuelpillai U, Abumaree MH, Pertile MD, et al. Ectopic Bone Formation by Mesenchymal Stem Cells Derived from Human Term Placenta and the Decidua. Matsusaki M, editor. PLoS One. 2015 Oct 20;10(10):e0141246. [Google Scholar] [PubMed]
- 104.Li F, Chen Y-Z, Miao Z-N, Zheng S, Jin J. Human Placenta-Derived Mesenchymal Stem Cells with Silk Fibroin Biomaterial in the Repair of Articular Cartilage Defects. Cell Reprogram. 2012 Aug;14(4):334–41. [Google Scholar] [PubMed]
- 105.Wei JP, Nawata M, Wakitani S, Kametani K, Ota M, Toda A, et al. Human Amniotic Mesenchymal Cells Differentiate into Chondrocytes. Cloning Stem Cells. 2009 Mar;11(1):19–26. [Google Scholar] [PubMed]
- 106.Phunikom N, Boonmuen N, Kheolamai P, Suksen K, Manochantr S, Tantrawatpan C, et al. Andrographolide promotes proliferative and osteogenic potentials of human placenta-derived mesenchymal stem cells through the activation of Wnt/β-catenin signaling. Stem Cell Res Ther. 2021 Dec 14;12(1):241. [Google Scholar] [PubMed]
- 107.Hugot J-P, Chamaillard M, Zouali H, Lesage S, Cézard J-P, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001 May;411(6837):599–603. [Google Scholar] [PubMed]
- 108.Song J, Kang HJ, Hong JS, Kim CJ, Shim J-Y, Lee CW, et al. Umbilical cord-derived mesenchymal stem cell extracts reduce colitis in mice by re-polarizing intestinal macrophages. Sci Rep. 2017 Dec 25;7(1):9412. [Google Scholar] [PubMed]
- 109.Mayer L, Pandak WM, Melmed GY, Hanauer SB, Johnson K, Payne D, et al. Safety and Tolerability of Human Placenta-Derived Cells (PDA001) in Treatment-Resistant Crohn’s Disease. Inflamm Bowel Dis. 2013 Mar;19(4):754–60. [Google Scholar] [PubMed]
- 110.Zhang J, Lv S, Liu X, Song B, Shi L. Umbilical Cord Mesenchymal Stem Cell Treatment for Crohn’s Disease: A Randomized Controlled Clinical Trial. Gut Liver. 2018 Jan 15;12(1):73–8. [Google Scholar] [PubMed]
- 111.What Are Clinical Trials and Studies? National Institute on Aging. https://www.nia.nih.gov/health/what- are-clinical-trials-and-studies. Accessed on 5 October 2021. [Google Scholar] [PubMed]

